
Concrete is a composite material composed of aggregate bonded together with a fluid cement that cures to a solid over time. Concrete is the second-most-used substance in the world after water, and is the most widely used building material. Its usage worldwide, ton for ton, is twice that of steel, wood, plastics, and aluminium combined.

Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, D. Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.

Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that greatly increase the surface area available for adsorption or chemical reactions that can be thought of as a microscopic "sponge" structure. Activation is analogous to making popcorn from dried corn kernels: popcorn is light, fluffy, and its kernels have a high surface-area-to-volume ratio. Activated is sometimes replaced by active.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.

Soil mechanics is a branch of soil physics and applied mechanics that describes the behavior of soils. It differs from fluid mechanics and solid mechanics in the sense that soils consist of a heterogeneous mixture of fluids and particles but soil may also contain organic solids and other matter. Along with rock mechanics, soil mechanics provides the theoretical basis for analysis in geotechnical engineering, a subdiscipline of civil engineering, and engineering geology, a subdiscipline of geology. Soil mechanics is used to analyze the deformations of and flow of fluids within natural and man-made structures that are supported on or made of soil, or structures that are buried in soils. Example applications are building and bridge foundations, retaining walls, dams, and buried pipeline systems. Principles of soil mechanics are also used in related disciplines such as geophysical engineering, coastal engineering, agricultural engineering, and hydrology.

Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina. The particles are plate-shaped with an average diameter around 1 μm and a thickness of 0.96 nm; magnification of about 25,000 times, using an electron microscope, is required to resolve individual clay particles. Members of this group include saponite, nontronite, beidellite, and hectorite.

Water content or moisture content is the quantity of water contained in a material, such as soil, rock, ceramics, crops, or wood. Water content is used in a wide range of scientific and technical areas, and is expressed as a ratio, which can range from 0 to the value of the materials' porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis.
Brunauer–Emmett–Teller (BET) theory aims to explain the physical adsorption of gas molecules on a solid surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of materials. The observations are very often referred to as physical adsorption or physisorption. In 1938, Stephen Brunauer, Paul Hugh Emmett, and Edward Teller presented their theory in the Journal of the American Chemical Society. BET theory applies to systems of multilayer adsorption that usually utilizes a probing gas (called the adsorbate) that does not react chemically with the adsorptive (the material upon which the gas attaches to) to quantify specific surface area. Nitrogen is the most commonly employed gaseous adsorbate for probing surface(s). For this reason, standard BET analysis is most often conducted at the boiling temperature of N2 (77 K). Other probing adsorbates are also utilized, albeit less often, allowing the measurement of surface area at different temperatures and measurement scales. These include argon, carbon dioxide, and water. Specific surface area is a scale-dependent property, with no single true value of specific surface area definable, and thus quantities of specific surface area determined through BET theory may depend on the adsorbate molecule utilized and its adsorption cross section.
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors. In the early 1870s a consulting chemist to the Royal Agricultural Society in England, named J. Thomas Way, performed many experiments on how soils exchange ions, and is considered the father of soil chemistry. Other scientists who contributed to this branch of ecology include Edmund Ruffin, and Linus Pauling.

A sieve analysis is a practice or procedure used in geology, civil engineering, and chemical engineering to assess the particle size distribution of a granular material by allowing the material to pass through a series of sieves of progressively smaller mesh size and weighing the amount of material that is stopped by each sieve as a fraction of the whole mass.
The pore space of soil contains the liquid and gas phases of soil, i.e., everything but the solid phase that contains mainly minerals of varying sizes as well as organic compounds.
Dynamic vapor sorption (DVS) is a gravimetric technique that measures how quickly and how much of a solvent is absorbed by a sample such as a dry powder absorbing water. It does this by varying the vapor concentration surrounding the sample and measuring the change in mass which this produces. The technique is mostly used for water vapor, but is suitable for a wide range of organic solvents. Daryl Williams, founder of Surface Measurement Systems Ltd, developed Dynamic Vapor Sorption in 1991; the first instrument was delivered to Pfizer UK in 1992. DVS was originally developed to replace the time and labor-intensive desiccators and saturated salt solutions used to measure water vapor sorption isotherms.
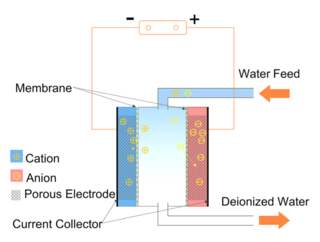
Capacitive deionization (CDI) is a technology to deionize water by applying an electrical potential difference over two electrodes, which are often made of porous carbon. In other words, CDI is an electro-sorption method using a combination of a sorption media and an electrical field to separate ions and charged particles. Anions, ions with a negative charge, are removed from the water and are stored in the positively polarized electrode. Likewise, cations are stored in the cathode, which is the negatively polarized electrode.
Concrete has relatively high compressive strength, but significantly lower tensile strength. The compressive strength is typically controlled with the ratio of water to cement when forming the concrete, and tensile strength is increased by additives, typically steel, to create reinforced concrete. In other words we can say concrete is made up of sand, ballast, cement and water.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.

Particle deposition is the spontaneous attachment of particles to surfaces. The particles in question are normally colloidal particles, while the surfaces involved may be planar, curved, or may represent particles much larger in size than the depositing ones. Deposition processes may be triggered by appropriate hydrodynamic flow conditions and favorable particle-surface interactions. Depositing particles may just form a monolayer which further inhibits additional particle deposition, and thereby one refers to surface blocking. Initially attached particles may also serve as seeds for further particle deposition, which leads to the formation of thicker particle deposits, and this process is termed as surface ripening or fouling. While deposition processes are normally irreversible, initially deposited particles may also detach. The latter process is known as particle release and is often triggered by the addition of appropriate chemicals or a modification in flow conditions.

Soil aggregate stability is a measure of the ability of soil aggregates—soil particles that bind together—to resist breaking apart when exposed to external forces such as water erosion and wind erosion, shrinking and swelling processes, and tillage. Soil aggregate stability is a measure of soil structure and can be affected by soil management.
3- Neville, A. M. “Properties of Concrete”, 4th & Final ed., Longman, Malaysia, 1995 rep. 1996, 844 pp.
4 - Field usage information from a manufacturer - https://blog.kryton.com/2012/08/what-ssd/














