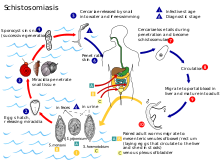
Schistosomiasis, also known as snail fever, bilharzia, and Katayama fever, is a disease caused by parasitic flatworms called schistosomes. The urinary tract or the intestines may be infected. Symptoms include abdominal pain, diarrhea, bloody stool, or blood in the urine. Those who have been infected for a long time may experience liver damage, kidney failure, infertility, or bladder cancer. In children, it may cause poor growth and learning difficulty.

Trematoda is a class of flatworms known as flukes or trematodes. They are obligate internal parasites with a complex life cycle requiring at least two hosts. The intermediate host, in which asexual reproduction occurs, is usually a snail. The definitive host, where the flukes sexually reproduce, is a vertebrate. Infection by trematodes can cause disease in all five traditional vertebrate classes: mammals, birds, amphibians, reptiles, and fish.
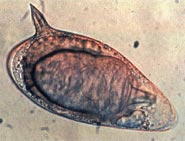
Schistosoma is a genus of trematodes, commonly known as blood flukes. They are parasitic flatworms responsible for a highly significant group of infections in humans termed schistosomiasis, which is considered by the World Health Organization as the second-most socioeconomically devastating parasitic disease, with hundreds of millions infected worldwide.
Schistosoma japonicum is an important parasite and one of the major infectious agents of schistosomiasis. This parasite has a very wide host range, infecting at least 31 species of wild mammals, including nine carnivores, 16 rodents, one primate (human), two insectivores and three artiodactyls and therefore it can be considered a true zoonosis. Travelers should be well-aware of where this parasite might be a problem and how to prevent the infection. S. japonicum occurs in the Far East, such as China, the Philippines, Indonesia and Southeast Asia.

Schistosoma mansoni is a water-borne parasite of humans, and belongs to the group of blood flukes (Schistosoma). The adult lives in the blood vessels near the human intestine. It causes intestinal schistosomiasis. Clinical symptoms are caused by the eggs. As the leading cause of schistosomiasis in the world, it is the most prevalent parasite in humans. It is classified as a neglected tropical disease. As of 2021, the World Health Organization reports that 251.4 million people have schistosomiasis and most of it is due to S. mansoni. It is found in Africa, the Middle East, the Caribbean, Brazil, Venezuela and Suriname.

Schistosoma intercalatum is a parasitic worm found in parts of western and central Africa. There are two strains: the Lower Guinea strain and the Zaire strain. S. intercalatum is one of the major agents of the rectal form of schistosomiasis, also called bilharzia. It is a trematode, and being part of the genus Schistosoma, it is commonly referred to as a blood-fluke since the adult resides in blood vessels.

Schistosoma haematobium is a species of digenetic trematode, belonging to a group (genus) of blood flukes (Schistosoma). It is found in Africa and the Middle East. It is the major agent of schistosomiasis, the most prevalent parasitic infection in humans. It is the only blood fluke that infects the urinary tract, causing urinary schistosomiasis, and is the leading cause of bladder cancer. The diseases are caused by the eggs.

Theodor Maximilian Bilharz was a German physician who made pioneering discoveries in the field of parasitology. His contributions led to the foundation of tropical medicine. He is best remembered as the discoverer of the blood fluke Schistosoma haematobium, the causative parasite of bloody urine (haematuria) known since ancient times in Egypt. The parasite, as the cause of bladder cancer, is declared by the International Agency for Research on Cancer as Group 1 carcinogen. The infection is known by an eponymous term bilharzia or bilharziasis, as well as by schistosomiasis.

Trematocranus placodon is a species of cichlid fish endemic to Lake Malawi, Lake Malombe and the upper reaches of the Shire River in Africa. It is mainly a shallow-water species that prefers to occupy areas with patches of Vallisneria, but it can occur as deep as 31 m (102 ft). It can reach a total length of up to 25 cm (9.8 in).
Schistosoma indicum is a species of digenetic trematode in the family Schistosomatidae. The parasite is widespread in domestic animals in India and other Asian countries.

A Schistosomiasis vaccine is a vaccine against Schistosomiasis, a parasitic disease caused by several species of fluke of the genus Schistosoma. No effective vaccine for the disease exists yet. Schistosomiasis affects over 200 million people worldwide, mainly in rural agricultural and peri-urban areas of the third world, and approximately 10% suffer severe health complications from the infection. While chemotherapeutic drugs, such as praziquantel, oxamniquine and metrifonate both no longer on the market, are currently considered safe and effective for the treatment of schistosomiasis, reinfection occurs frequently following drug treatment, thus a vaccine is sought to provide long-term treatment. Additionally, experimental vaccination efforts have been successful in animal models of schistosomiasis.
Schistosoma mekongi is a species of trematodes, also known as flukes. It is one of the five major schistosomes that account for all human infections, the other four being S. haematobium, S. mansoni, S. japonicum, and S. intercalatum. This trematode causes schistosomiasis in humans.

Indoplanorbis is a genus of air-breathing freshwater snail. Its only member species is Indoplanorbis exustus, an aquatic pulmonate gastropod mollusk in the family Planorbidae, the ram's horn snails. The species is widely distributed across the tropics. It serves as an important intermediate host for several trematode parasites. The invasive nature and ecological tolerance of Indoplanorbis exustus add to its importance in veterinary and medical science.

Schistosoma spindale is a species of digenetic trematode in the family Schistosomatidae. It causes intestinal schistosomiasis in the ruminants.

Bulinus forskalii is a species of tropical freshwater snail with a sinistral shell, an aquatic gastropod mollusk in the family Bulinidae, the ramshorn snails and their allies.
Bulinus nasutus is a species of tropical freshwater snail with a sinistral shell, an aquatic gastropod mollusk in the family Planorbidae, the ramshorn snails and their allies.

Bivitellobilharzia nairi is a species of trematodes, part of the family Schistosomatidae. This is a fairly new identified endoparasite that was found in 1945 by Mudaliar and Ramanujachari, who first recorded the parasite in India. Researchers collected fecal samples of the Indian rhinoceros and were startled to find B. nairi eggs.

The Cavu or rivière de Cavu, is a short river in the Corse-du-Sud department of Corsica which discharges into the Tyrrhenian Sea, and the Mediterranean Sea. In 2014 the Cavu became the first place of re-emerging schistosomiasis in Europe. As of 2016 120 people have become infected after bathing in it.
Carcinogenic parasites are parasitic organisms that depend on other organisms for their survival, and cause cancer in such hosts. Three species of flukes (trematodes) are medically-proven carcinogenic parasites, namely the urinary blood fluke, the Southeast Asian liver fluke and the Chinese liver fluke. S. haematobium is prevalent in Africa and the Middle East, and is the leading cause of bladder cancer. O. viverrini and C. sinensis are both found in eastern and southeastern Asia, and are responsible for cholangiocarcinoma. The International Agency for Research on Cancer declared them in 2009 as a Group 1 biological carcinogens in humans.

Planorbarius metidjensis is a freshwater lung snail.