Related Research Articles

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees.
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets.

DC-SIGN also known as CD209 is a protein which in humans is encoded by the CD209 gene.
Cyanovirin-N (CV-N) is a protein produced by the cyanobacterium Nostoc ellipsosporum that displays virucidal activity against several viruses, including human immunodeficiency virus (HIV). The virucidal activity of CV-N is mediated through specific high-affinity interactions with the viral surface envelope glycoproteins gp120 and gp41, as well as to high-mannose oligosaccharides found on the HIV envelope. In addition, CV-N is active against rhinoviruses, human parainfluenza virus, respiratory syncytial virus, and enteric viruses. The virucidal activity of CV-N against influenza virus is directed towards viral haemagglutinin. CV-N has a complex fold composed of a duplication of a tandem repeat of two homologous motifs comprising three-stranded beta-sheet and beta-hairpins.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.

Langerin (CD207) is a type II transmembrane protein which is encoded by the CD207 gene in humans. It was discovered by scientists Sem Saeland and Jenny Valladeau as a main part of Birbeck granules. Langerin is C-type lectin receptor on Langerhans cells (LCs) and in mice also on dermal interstitial CD103+ dendritic cells (DC) and on resident CD8+ DC in lymph nodes.
In enzymology, a cholesterol 25-hydroxylase (EC 1.14.99.38) is an enzyme that catalyzes the chemical reaction

C-type lectin domain family 4 member M is a protein that in humans is encoded by the CLEC4M gene. CLEC4M has also been designated as CD299.

Griffithsin is a protein isolated from the red algae Griffithsia. It has a 121-amino acid sequence which exhibits a Jacalin-like lectin fold. Several structures of this protein have been solved by X-ray crystallography and deposited in the PDB. It has been shown in vitro to be a highly potent HIV entry inhibitor. It is currently being investigated as a potential microbicide for use in the prevention of the transmission of HIV.

Protein ERGIC-53 also known as ER-Golgi intermediate compartment 53 kDa protein or lectin mannose-binding 1 is a protein that in humans is encoded by the LMAN1 gene.

DNA dC->dU-editing enzyme APOBEC-3C is a protein that in humans is encoded by the APOBEC3C gene.

Tetherin, also known as bone marrow stromal antigen 2, is a lipid raft associated protein that in humans is encoded by the BST2 gene. In addition, tetherin has been designated as CD317. This protein is constitutively expressed in mature B cells, plasma cells and plasmacytoid dendritic cells, and in many other cells, it is only expressed as a response to stimuli from IFN pathway.

FGI-104 is the name of an experimental broad-spectrum antiviral drug, with activity against a range of viruses including hepatitis B, hepatitis C, HIV, Ebola virus, and Venezuelan equine encephalitis virus.
BanLec is a lectin from the jacalin-related lectin family isolated from the fruit of the bananas Musa acuminata and Musa balbisiana. BanLec is one of the predominant proteins in the pulp of ripe bananas and has binding specificity for mannose and mannose-containing oligosaccharides. A 2010 study reported that BanLec was a potent inhibitor of HIV replication.

Antiviral proteins are proteins that are induced by human or animal cells to interfere with viral replication. These proteins are isolated to inhibit the virus from replicating in a host's cells and stop it from spreading to other cells. The Pokeweed antiviral protein and the Zinc-Finger antiviral protein are two major antiviral proteins that have undergone several tests for viruses, including HIV and influenza.
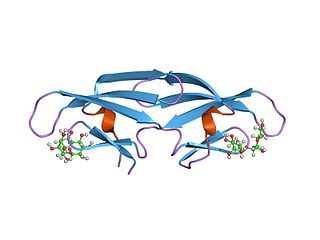
In molecular biology, the CVNH domain is a conserved protein domain. It is found in the sugar-binding antiviral protein cyanovirin-N (CVN) as well as proteins from filamentous ascomycetes and in the fern Ceratopteris richardii.

In molecular biology, the jacalin-like lectin domain is a mannose-binding lectin domain with a beta-prism fold consisting of three 4-stranded beta-sheets, with an internal pseudo 3-fold symmetry. Some lectins in this group stimulate distinct T- and B-cell functions, such as Jacalin, which binds to the T-antigen and acts as an agglutinin. This domain is found in 1 to 6 copies in lectins. The domain is also found in the salt-stress induced protein from rice and an animal prostatic spermine-binding protein.
Scytonema varium is a cultured cyanobacterium of the genus Scytonema. It is one of many anti viral protein producing algae. In a similar manner to Cyanovirin-N from Nostoc Ellipsosporum and griffithsin from the red algae Griffithsia, Scytonema varium secretes the broad-spectrum antiviral protein scytovirin which can inactivate both the HIV virus, and Ebola virus, offering hope of treatment for many diseases with viral etiology (cause). It is currently being investigated as a topical microbicide for HIV prophylaxis.

Galidesivir is an antiviral drug, an adenosine analog. It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.
Paul Darren Bieniasz is a British-American virologist whose main area of research is HIV/AIDS. He is currently a professor of retrovirology at the Rockefeller University. He received the 2015 KT Jeang Retrovirology Prize and the 2010 Eli Lilly and Company Research Award. Bieniasz has been a Howard Hughes Medical Institute investigator since 2008.
References
- ↑ Bokesch HR, O'Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, Turpin J, Watson K, Buckheit RW, Boyd MR (March 2003). "A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium". Biochemistry. 42 (9): 2578–84. doi:10.1021/bi0205698. PMID 12614152.
- ↑ Xiong C, O'Keefe BR, Botos I, Wlodawer A, McMahon JB (April 2006). "Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium". Protein Expression and Purification. 46 (2): 233–9. doi:10.1016/j.pep.2005.09.019. PMID 16289703.
- ↑ McFeeters RL, Xiong C, O'Keefe BR, Bokesch HR, McMahon JB, Ratner DM, Castelli R, Seeberger PH, Byrd RA (June 2007). "The novel fold of scytovirin reveals a new twist for antiviral entry inhibitors". Journal of Molecular Biology. 369 (2): 451–61. doi:10.1016/j.jmb.2007.03.030. PMC 2696897 . PMID 17434526.
- ↑ Moulaei T, Stuchlik O, Reed M, Yuan W, Pohl J, Lu W, Haugh-Krumpe L, O'Keefe BR, Wlodawer A (September 2010). "Topology of the disulfide bonds in the antiviral lectin scytovirin". Protein Science. 19 (9): 1649–61. doi:10.1002/pro.445. PMC 2975129 . PMID 20572021.
- ↑ Ziółkowska NE, Wlodawer A (2006). "Structural studies of algal lectins with anti-HIV activity". Acta Biochimica Polonica. 53 (4): 617–26. doi: 10.18388/abp.2006_3290 . PMID 17128290.
- ↑ Li Y, Zhang X, Chen G, Wei D, Chen F (2008). "Algal lectins for potential prevention of HIV transmission". Current Medicinal Chemistry. 15 (11): 1096–104. doi:10.2174/092986708784221421. PMID 18473805.
- ↑ Garrison AR, Giomarelli BG, Lear-Rooney CM, Saucedo CJ, Yellayi S, Krumpe LR, Rose M, Paragas J, Bray M, Olinger GG, McMahon JB, Huggins J, O'Keefe BR (December 2014). "The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus". Antiviral Research. 112: 1–7. doi:10.1016/j.antiviral.2014.09.012. PMC 4258435 . PMID 25265598.
- ↑ Barton C, Kouokam JC, Lasnik AB, Foreman O, Cambon A, Brock G, Montefiori DC, Vojdani F, McCormick AA, O'Keefe BR, Palmer KE (2014). "Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models". Antimicrobial Agents and Chemotherapy. 58 (1): 120–7. doi:10.1128/AAC.01407-13. PMC 3910741 . PMID 24145548.
- ↑ Huskens D, Schols D (July 2012). "Algal lectins as potential HIV microbicide candidates". Marine Drugs. 10 (7): 1476–97. doi: 10.3390/md10071476 . PMC 3407925 . PMID 22851920.