Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds. Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness. Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.

Green tea is a type of tea that is made from Camellia sinensis leaves and buds that have not undergone the same withering and oxidation process which is used to make oolong teas and black teas. Green tea originated in China, and since then its production and manufacture has spread to other countries in East Asia.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Nootropics are numerous natural, semi-synthetic and synthetic molecules that improve cognitive functions.
Although health benefits have been assumed throughout the history of using Camellia sinensis as a common beverage, there is no high-quality evidence that consuming tea confers significant benefits other than possibly increasing alertness, an effect caused by caffeine in the tea leaves. In clinical research conducted over the early 21st century, tea has been studied extensively for its potential to lower the risk of human diseases, but there is no good scientific evidence to indicate that consuming tea affects any disease or improves health.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
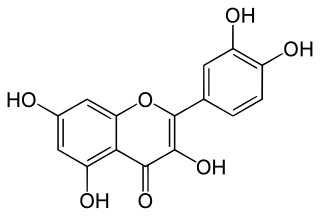
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
Polyphenon is a series of high grade green tea polyphenol extracts manufactured by the Mitsui Norin Co., Ltd. of Japan. The extracts are in part the result of a water based extraction method which begins with green tea leaves, and then involves successive steps which concentrate the catechins thought to be responsible for the health benefits of green tea.

Hydroxytyrosol is an organic compound with the formula (HO)2C6H3CH2CH2OH. Classified as a phenylethanoid, i.e. a relative of phenethyl alcohol. Its derivatives are found in a variety of natural sources, notably olive oils and wines. Hydroxytyrosol is a colorless solid, although samples often turn beige during storage. It is a derivative, formally speaking, of catechol.
Thearubigins are polymeric polyphenols that are formed during the enzymatic oxidation and condensation of two gallocatechins with the participation of polyphenol oxidases during the fermentation reactions in black tea. Thearubigins are red in colour and are responsible for much of the staining effect of tea. Therefore, a black tea often appears red while a green or white tea has a much clearer appearance. The colour of a black tea, however, is affected by many other factors as well, such as the amount of theaflavins, another oxidized form of polyphenols.

Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position.

Theaflavin digallate (TFDG) is an antioxidant natural phenol found in black tea, and a theaflavin derivative.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Gallocatechin gallate (GCG) is the ester of gallocatechin and gallic acid and a type of catechin. It is an epimer of epigallocatechin gallate (EGCG).

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.