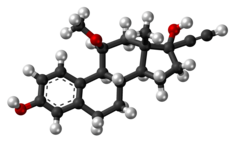
Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disorders, and certain hormone-sensitive cancers. It is usually taken by mouth but is also used as a patch and vaginal ring.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Gestrinone, sold under the brand names Dimetrose and Nemestran among others, is a medication which is used in the treatment of endometriosis. It has also been used to treat other conditions such as uterine fibroids and heavy menstrual bleeding and has been investigated as a method of birth control. Gestrinone is used alone and is not formulated in combination with other medications. It is taken by mouth or in through the vagina.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.

Mestranol, sold under the brand names Enovid, Norinyl, and Ortho-Novum among others, is an estrogen medication which has been used in birth control pills, menopausal hormone therapy, and the treatment of menstrual disorders. It is formulated in combination with a progestin and is not available alone. It is taken by mouth.

Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis. It is also used in menopausal hormone therapy and to treat heavy periods. Dienogest is available both alone and in combination with estrogens. It is taken by mouth.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Epiestriol, or epioestriol, also known as 16β-epiestriol or simply 16-epiestriol as well as 16β-hydroxy-17β-estradiol, is a minor and weak endogenous estrogen, and the 16β-epimer of estriol. Epiestriol is used clinically in the treatment of acne. In addition to its estrogenic actions, epiestriol has been found to possess significant anti-inflammatory properties without glycogenic activity or immunosuppressive effects, an interesting finding that is in contrast to conventional anti-inflammatory steroids like hydrocortisone.

17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol. It is formed from 16α-hydroxyestrone. In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ. It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα. 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

17α-Ethynyl-3α-androstanediol, also known as 17α-ethynyl-5α-androstane-3α,17β-diol, is a synthetic androstane steroid and a 17α-substituted derivative of 3α-androstanediol which was never marketed. It was under development for the treatment of prostate cancer but was discontinued.

RU-16117 is an estrogen medication which was investigated for the potential treatment of symptoms of estrogen deficiency such as hot flashes and osteoporosis in women but was never marketed. It was developed for use by mouth.

7α-Methylestradiol (7α-Me-E2), also known as 7α-methylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrogen and an active metabolite of the androgen/anabolic steroids trestolone/Methandienone. It is considered to be responsible for the estrogenic activity of trestolone. The compound shows about the same affinity for the estrogen receptor as estradiol.

5α-Dihydroethisterone is an active metabolite of the formerly clinically used but now-discontinued progestin ethisterone and the experimental and never-marketed hormonal antineoplastic agent ethynylandrostanediol (HE-3235). Its formation from its parent drugs is catalyzed by 5α-reductase in tissues that express the enzyme in high amounts like the liver, skin, hair follicles, and prostate gland. 5α-DHET has significant affinity for steroid hormone receptors and may contribute importantly to the activities of its parent drugs.

17α-Ethynyl-3β-androstanediol is a synthetic estrogen and a 17α-substituted derivative of 3β-androstanediol which was never marketed.

11β-Methoxyestradiol is a synthetic steroidal estrogen which was never marketed. It has about 86% of the relative binding affinity of estradiol for the estrogen receptor. 11β-MeOE2 is structurally related to moxestrol (11β-methoxy-17α-ethynylestradiol). 11β-MeOE2 and moxestrol are substantially more potent than their non-methoxylated analogues in mice.