Metenolone enanthate, or methenolone enanthate, sold under the brand names Primobolan Depot and Nibal Injection, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of anemia due to bone marrow failure. It is given by injection into muscle. Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly only available on the black market. A related drug, metenolone acetate, is taken by mouth.

Etynodiol diacetate, or ethynodiol diacetate, sold under the brand names Demulen and Femulen among others, is a progestin medication which is used in birth control pills. The medication is available only in combination with an estrogen. It is taken by mouth.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Mibolerone, also known as dimethylnortestosterone (DMNT) and sold under the brand names Cheque Drops and Matenon, is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was marketed by Upjohn for use as a veterinary drug. It was indicated specifically as an oral treatment for prevention of estrus (heat) in adult female dogs.

Drostanolone propionate, or dromostanolone propionate, sold under the brand names Drolban, Masteril, and Masteron among others, is an androgen and anabolic steroid (AAS) medication which was used to treat breast cancer in women but is now no longer marketed. It is given by injection into muscle.

Mestanolone, also known as methylandrostanolone and sold under the brand names Androstalone and Ermalone among others, is an androgen and anabolic steroid (AAS) medication which is mostly no longer used. It is still available for use in Japan however. It is taken by mouth.
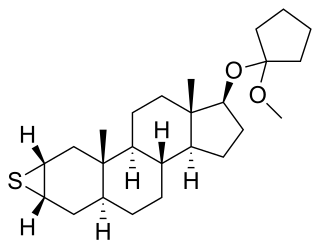
Mepitiostane, sold under the brand name Thioderon, is an orally active antiestrogen and anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which is marketed in Japan as an antineoplastic agent for the treatment of breast cancer. It is a prodrug of epitiostanol. The drug was patented and described in 1968.

Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was available both alone and in combination with an estrogen. The medication is taken by mouth.

Benzestrol is a synthetic nonsteroidal estrogen of the stilbestrol group which was formerly used medically but has since been discontinued. The stilbestrol estrogens, the best-known of which is diethylstilbestrol (DES) were used extensively in the mid-1900s and were finally banned by the FDA due to them causing tumors in the children of women who used them.

Quinestradol, also known as quinestradiol or quinestriol, as well as estriol 3-cyclopentyl ether (E3CPE), is a synthetic estrogen and estrogen ether which is no longer marketed. It is the 3-cyclopentyl ether of estriol. The medication has been studied in the treatment of stress incontinence in elderly women, with effectiveness observed.
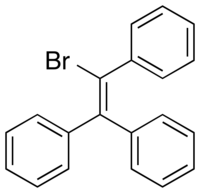
Broparestrol (INN), also known as α-bromo-α,β-diphenyl-β-p-ethylphenylethylene (BDPE), is a synthetic, nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that has been used in Europe as a dermatological agent and for the treatment of breast cancer. The drug is described as slightly estrogenic and potently antiestrogenic, and inhibits mammary gland development and suppresses prolactin levels in animals. It is structurally related to clomifene and diethylstilbestrol. Broparestrol is a mixture of E- and Z- isomers, both of which are active and are similarly antiestrogenic but, unlike broparestrol, were never marketed.
Nilestriol (INN), also known as nylestriol, is a synthetic estrogen which was patented in 1971 and is marketed in China. It is the 3-cyclopentyl ether of ethinylestriol, and is also known as ethinylestriol cyclopentyl ether (EE3CPE). Nilestriol is a prodrug of ethinylestriol, and is a more potent estrogen in comparison. It is described as a slowly-metabolized, long-acting estrogen and derivative of estriol. Nilestriol was assessed in combination with levonorgestrel for the potential treatment of postmenopausal osteoporosis, but this formulation ultimately was not marketed.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.

Hydroxyestrone diacetate, or 16α-hydroxyestrone diacetate, also known as 3,16α-dihydroxyestra-1,3,5(10)-trien-17-one 3,16α-diacetate, is a synthetic, steroidal estrogen which has been marketed in France, Spain, Brazil, and Argentina. It is a derivative of 16α-hydroxyestrone with an acetate esters attached at the C3 and C16α positions.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.

Mestilbol, also known as diethylstilbestrol monomethyl ether, is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol. It was developed by Wallace & Tiernan Company, patented in 1940, and introduced for medical use in the 1940s, but is now no longer marketed. Mestilbol was available both as oral tablets and in oil for intramuscular injection. The drug is gradually demethylated in the body into diethylstilbestrol and hence is a prodrug of diethylstilbestrol. Mestilbol is a highly active estrogen, although somewhat less so than diethylstilbestrol, but is longer-lasting in comparison.
Phenestrol, or fenestrol, also known as hexestrol bis[4-[bis(2-chloroethyl)amino]phenylacetate, is a synthetic, nonsteroidal estrogen and cytostatic antineoplastic agent and a chlorphenacyl nitrogen mustard ester of hexestrol which was developed in the early 1960s for the treatment of hormone-dependent tumors but was never marketed.
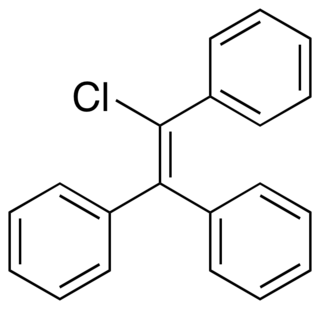
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Allenestrol, or allenoestrol, also known as α,α-dimethyl-β-ethylallenolic acid or as methallenestrilphenol, is a synthetic, nonsteroidal estrogen and a derivative of allenolic acid that was never marketed. A methyl ether of allenestrol, methallenestril (methallenestrol), is also an estrogen, but, in contrast to allenestrol, has been marketed.