 | |
| Clinical data | |
|---|---|
| Trade names | Digalen, Digitaline, Digitmerck, others |
| Routes of administration | By mouth, Intravenous injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 98–100% (oral) |
| Protein binding | 90–97% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 7–8 days |
| Excretion | 60% via urine, 40% via faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.691 |
| Chemical and physical data | |
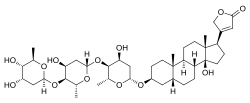
| Formula | C41H64O13 |
| Molar mass | 764.950 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Digitoxin is a cardiac glycoside used for the treatment of heart failure and certain kinds of heart arrhythmia. It is a phytosteroid and is similar in structure and effects to digoxin, though the effects are longer-lasting. Unlike digoxin, which is eliminated from the body via the kidneys, it is eliminated via the liver, and so can be used in patients with poor or erratic kidney function. While several controlled trials have shown digoxin to be effective in a proportion of patients treated for heart failure, the evidence base for digitoxin is not as strong, although it is presumed to be similarly effective. [1]