α-Ketoglutaric acid is a keto acid.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
Glutamine synthetase (GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:
Glutamate transporters are a family of neurotransmitter transporter proteins that move glutamate – the principal excitatory neurotransmitter – across a membrane. The family of glutamate transporters is composed of two primary subclasses: the excitatory amino acid transporter (EAAT) family and vesicular glutamate transporter (VGLUT) family. In the brain, EAATs remove glutamate from the synaptic cleft and extrasynaptic sites via glutamate reuptake into glial cells and neurons, while VGLUTs move glutamate from the cell cytoplasm into synaptic vesicles. Glutamate transporters also transport aspartate and are present in virtually all peripheral tissues, including the heart, liver, testes, and bone. They exhibit stereoselectivity for L-glutamate but transport both L-aspartate and D-aspartate.

Sodium phenylbutyrate, sold under the brand name Buphenyl among others, is a salt of an aromatic fatty acid, 4-phenylbutyrate (4-PBA) or 4-phenylbutyric acid. The compound is used to treat urea cycle disorders, because its metabolites offer an alternative pathway to the urea cycle to allow excretion of excess nitrogen.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

Glutaminase is an amidohydrolase enzyme that generates glutamate from glutamine. Glutaminase has tissue-specific isoenzymes. Glutaminase has an important role in glial cells.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Guanosine monophosphate synthetase, also known as GMPS is an enzyme that converts xanthosine monophosphate to guanosine monophosphate.

In enzymology, an aminodeoxychorismate synthase is an enzyme that catalyzes the chemical reaction

In enzymology, a dihydrofolate synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a tetrahydrofolate synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-isopropylmalate synthase (EC 2.3.3.13) is an enzyme that catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a [glutamate—ammonia-ligase] adenylyltransferase is an enzyme that catalyzes the chemical reaction

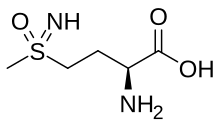
Peptide methionine sulfoxide reductase (Msr) is a family of enzymes that in humans is encoded by the MSRA gene.

CTP synthase 2 is an enzyme that in humans is encoded by the CTPS2 gene.

5′-Phosphoribosyl-5-aminoimidazole is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from AIR. It is an intermediate in the adenine pathway and is synthesized from 5′-phosphoribosylformylglycinamidine by AIR synthetase.
Peptide-methionine (S)-S-oxide reductase (EC 1.8.4.11, MsrA, methionine sulphoxide reductase A, methionine S-oxide reductase (S-form oxidizing), methionine sulfoxide reductase A, peptide methionine sulfoxide reductase, formerly protein-methionine-S-oxide reductase) is an enzyme with systematic name peptide-L-methionine:thioredoxin-disulfide S-oxidoreductase (L-methionine (S)-S-oxide-forming). This enzyme catalyses the following chemical reaction
TRNA (adenine58-N1)-methyltransferase (EC 2.1.1.220, tRNA m1A58 methyltransferase, tRNA (m1A58) methyltransferase, TrmI, tRNA (m1A58) Mtase, Rv2118cp, Gcd10p-Gcd14p, Trm61p-Trm6p) is an enzyme with systematic name S-adenosyl-L-methionine:tRNA (adenine58-N1)-methyltransferase. This enzyme catalyses the following chemical reaction