gamma-Aminobutyric acid, or γ-aminobutyric acid, or GABA, is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

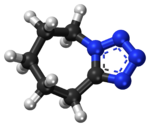
Bicuculline is a phthalide-isoquinoline compound that is a light-sensitive competitive antagonist of GABAA receptors. It was originally identified in 1932 in plant alkaloid extracts and has been isolated from Dicentra cucullaria, Adlumia fungosa, and several Corydalis species. Since it blocks the inhibitory action of GABA receptors, the action of bicuculline mimics epilepsy; it also causes convulsions. This property is utilized in laboratories around the world in the in vitro study of epilepsy, generally in hippocampal or cortical neurons in prepared brain slices from rodents. This compound is also routinely used to isolate glutamatergic receptor function.
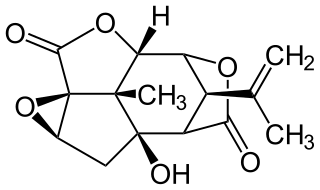
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. For instance, under physiological conditions Cl− will flow inside the cell if the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions if the receptor is activated. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).
Neuropharmacology is the study of how drugs affect cellular function in the nervous system, and the neural mechanisms through which they influence behavior. There are two main branches of neuropharmacology: behavioral and molecular. Behavioral neuropharmacology focuses on the study of how drugs affect human behavior (neuropsychopharmacology), including the study of how drug dependence and addiction affect the human brain. Molecular neuropharmacology involves the study of neurons and their neurochemical interactions, with the overall goal of developing drugs that have beneficial effects on neurological function. Both of these fields are closely connected, since both are concerned with the interactions of neurotransmitters, neuropeptides, neurohormones, neuromodulators, enzymes, second messengers, co-transporters, ion channels, and receptor proteins in the central and peripheral nervous systems. Studying these interactions, researchers are developing drugs to treat many different neurological disorders, including pain, neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease, psychological disorders, addiction, and many others.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.

An analeptic, in medicine, is a central nervous system stimulant. The term "analeptic" typically refers to respiratory analeptics. Analeptics are central nervous system (CNS) stimulants that include a wide variety of medications used to treat depression, attention deficit hyperactivity disorder (ADHD), and respiratory depression. Analeptics can also be used as convulsants, with low doses causing patients to experience heightened awareness, restlessness, and rapid breathing. The primary medical use of these drugs is as an anesthetic recovery tool or to treat emergency respiratory depression. Other drugs of this category are prethcamide, pentylenetetrazole, and nikethamide. Nikethamide is now withdrawn due to risk of convulsions. Analeptics have recently been used to better understand the treatment of a barbiturate overdose. Through the use of agents, researchers were able to treat obtundation and respiratory depression.

Tetramethylenedisulfotetramine (TETS) is an organic compound used as a rodenticide. It is an odorless, tasteless white powder that is slightly soluble in water, DMSO and acetone, and insoluble in methanol and ethanol. It is a sulfamide derivative. It can be synthesized by reacting sulfamide with formaldehyde under acidic condition. When crystallized from acetone, it forms cubic crystals with a melting point of 255–260 °C.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is used by injection into a vein over a 60-hour period under medical supervision.
GABA receptor antagonists are drugs that inhibit the action of GABA. In general these drugs produce stimulant and convulsant effects, and are mainly used for counteracting overdoses of sedative drugs.

Loreclezole is a sedative and an anticonvulsant which acts as a GABAA receptor positive allosteric modulator. The binding site of loreclezole has been shown experimentally to be shared by valerenic acid, an extract of the root of the valerian plant. Structurally, loreclezole is a triazole derivative. In animal seizure models, loreclezole is protective against pentylenetetrazol seizures but is less active in the maximal electroshock test. In addition, at low, nontoxic doses, the drug has anti-absence activity in a genetic model of generalized absence epilepsy. Consequently, loreclezole has a profile of activity similar to that of benzodiazepines. A potential benzodiazepine-like interaction with GABA receptors is suggested by the observation that the anticonvulsant effects of loreclezole can be reversed by benzodiazepine receptor inverse agonists. The benzodiazepine antagonist flumazenil, however, fails to alter the anticonvulsant activity of loreclezole, indicating that loreclezole is not a benzodiazepine receptor agonist. Using native rat and cloned human GABA-A receptors, loreclezole strongly potentiated GABA-activated chloride current. However, activity of the drug did not require the presence of the γ-subunit and was not blocked by flumazenil, confirming that loreclezole does not interact with the benzodiazepine recognition site.

Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.

A barbiturate is a drug that acts as a central nervous system depressant. Barbiturates are effective as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have largely been replaced by benzodiazepines and nonbenzodiazepines ("Z-drugs") in routine medical practice, particularly in the treatment of anxiety and insomnia, due to the significantly lower risk of addiction and overdose and the lack of an antidote for barbiturate overdose. Despite this, barbiturates are still in use for various purposes: in general anesthesia, epilepsy, treatment of acute migraines or cluster headaches, acute tension headaches, euthanasia, capital punishment, and assisted suicide.

PWZ-029 is a benzodiazepine derivative drug with nootropic effects developed by WiSys, It acts as a subtype-selective, mixed agonist-inverse agonist at the benzodiazepine binding site on the GABAA receptor, acting as a partial inverse agonist at the α5 subtype and a weak partial agonist at the α3 subtype. This gives it a mixed pharmacological profile, producing at low doses memory-enhancing effects but with no convulsant or anxiogenic effects or muscle weakness, although at higher doses it produces some sedative effects.

In pharmacology, GABAA receptor positive allosteric modulators are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
A channel modulator, or ion channel modulator, is a type of drug which modulates ion channels. They include channel blockers and channel openers.
Ionotropic GABA receptors (iGABARs) are ligand-gated ion channel of the GABA receptors class which are activated by gamma-aminobutyric acid (GABA), and include:
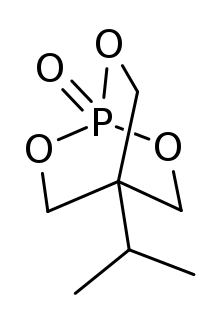
IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.
A GABAA receptor negative allosteric modulator is a negative allosteric modulator (NAM), or inhibitor, of the GABAA receptor, a ligand-gated ion channel of the major inhibitory neurotransmitter γ-aminobutyric acid (GABA). They are closely related and similar to GABAA receptor antagonists. The effects of GABAA receptor NAMs are functionally the opposite of those of GABAA receptor positive allosteric modulators (PAMs) like the benzodiazepines, barbiturates, and ethanol (alcohol). Non-selective GABAA receptor NAMs can produce a variety of effects including convulsions, neurotoxicity, and anxiety, among others.