An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Kava or kava kava is a crop of the Pacific Islands. The name kava is from Tongan and Marquesan, meaning 'bitter'; other names for kava include ʻawa (Hawaiʻi), ʻava (Samoa), yaqona or yagona (Fiji), sakau (Pohnpei), seka (Kosrae), and malok or malogu. Kava is consumed for its sedating effects throughout the Pacific Ocean cultures of Polynesia, including Hawaii and Vanuatu, Melanesia, some parts of Micronesia, such as Pohnpei and Kosrae, and the Philippines.

Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet. Kavalactones are under research for potential to have various psychotropic effects, including anxiolytic and sedative/hypnotic activities.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS). Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−).

Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. It was also used to murder women prisoners at Ravensbrück concentration camp. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Etifoxine, sold under the trade name Stresam among others, is a nonbenzodiazepine anxiolytic agent, primarily indicated for short-term management of adjustment disorder, specifically instances of situational depression accompanied by anxiety, such as stress-induced anxiety. Administration is by mouth. Side effects associated with etifoxine use include slight drowsiness, headache, skin eruptions, and allergic reactions. In rare cases, etifoxine has been linked to severe skin and liver toxicity, as well as menstrual bleeding between periods. Unlike benzodiazepines, etifoxine does not cause sedation or lack of coordination. Etifoxine acts as a GABAA receptor positive allosteric modulator and as a ligand for translocator proteins. Both mechanisms are conjectured to contribute to its anxiolytic properties.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.

Loreclezole is a sedative and an anticonvulsant which acts as a GABAA receptor positive allosteric modulator. The binding site of loreclezole has been shown experimentally to be shared by valerenic acid, an extract of the root of the valerian plant. Structurally, loreclezole is a triazole derivative. In animal seizure models, loreclezole is protective against pentylenetetrazol seizures but is less active in the maximal electroshock test. In addition, at low, nontoxic doses, the drug has anti-absence activity in a genetic model of generalized absence epilepsy. Consequently, loreclezole has a profile of activity similar to that of benzodiazepines. A potential benzodiazepine-like interaction with GABA receptors is suggested by the observation that the anticonvulsant effects of loreclezole can be reversed by benzodiazepine receptor inverse agonists. The benzodiazepine antagonist flumazenil, however, fails to alter the anticonvulsant activity of loreclezole, indicating that loreclezole is not a benzodiazepine receptor agonist. Using native rat and cloned human GABA-A receptors, loreclezole strongly potentiated GABA-activated chloride current. However, activity of the drug did not require the presence of the γ-subunit and was not blocked by flumazenil, confirming that loreclezole does not interact with the benzodiazepine recognition site.
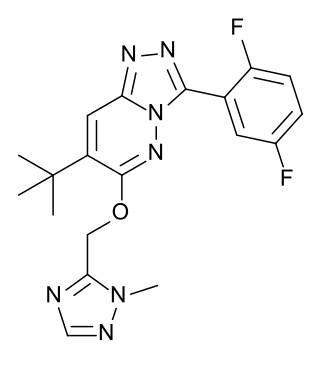
L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme.

ELB-139 (LS-191,811) is an anxiolytic drug with a novel chemical structure, which is used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

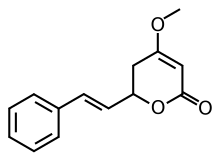
Desmethoxyyangonin or 5,6-dehydrokavain is one of the six main kavalactones found in the Piper methysticum (kava) plant.

Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also reported in Oroxylum indicum and Thyme. It is the aglycone of baicalin. Baicalein is one of the active ingredients of Sho-Saiko-To, which is a Chinese classic herbal formula, and listed in Japan as Kampo medicine.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.

CGP-7930 was the first positive allosteric modulator of GABAB receptors described in literature. CGP7930 is also a GABAA receptor positive allosteric modulator and a blocker of Potassium channels.
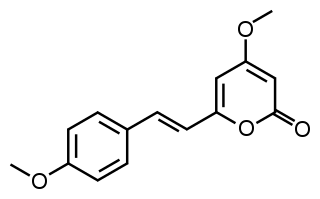
Yangonin is one of the six major kavalactones found in the kava plant. It has been shown to possess binding affinity for the cannabinoid receptor CB1 (Ki = 0.72 μM), and selectivity vs. the CB2 receptor (Ki >10 μM) where it behaves as an agonist. The CB1 receptor affinity of yangonin suggests that the endocannabinoid system might contribute to the complex human psychopharmacology of the traditional kava drink and the anxiolytic preparations obtained from the kava plant.

Dihydromethysticin is one of the six major kavalactones found in the kava plant.

Methysticin is one of the six major kavalactones found in the kava plant. Research suggests that methysticin and the related compound dihydromethysticin have CYP1A1 inducing effects which may be responsible for their toxicity. Additionally, methysticin has been shown to potentiate GABAA receptor activity, contributing to the overall anxiolytic profile of the kava plant.

A GABA analogue is a compound which is an analogue or derivative of the neurotransmitter gamma-Aminobutyric acid (GABA).
Deuterated etifoxine is a deuterated drug which is under development for the treatment of anxiety disorders and mood disorders. It was originated by GABA Therapeutics and is under development by GABA Therapeutics and ATAI Life Sciences. Deuterated etifoxine is a deuterated form of etifoxine (Stresam) with improved pharmacokinetic properties, for instance a longer elimination half-life and duration of action. Etifoxine has been widely used as an anxiolytic for many decades. Etifoxine and deuterated etifoxine are GABAA receptor positive allosteric modulators (GABAkines) and ligands of the translocator protein (TSPO), both of which may contribute to anxiolytic effects. The TSPO promotes steroidogenesis of inhibitory neurosteroids such as allopregnanolone, which act as potent GABAA receptor positive allosteric modulators, and hence interactions with the TSPO can also indirectly potentiate the GABAA receptor. The precise isotopic substitution of deuterated etifoxine has not yet been disclosed. As of January 2023, deuterated etifoxine is in phase 1 clinical trials for anxiety disorders and preclinical development for mood disorders.