The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
Chloroform, or trichloromethane, is an organic compound with the formula CHCl3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and PTFE. Chloroform is a trihalomethane that serves as a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water.

Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also recognised by the IUPAC) is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.

Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and abbreviations such as "perc", and "PCE", is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a mild sweet, sharp odor, detectable by most people at a concentration of 50 ppm.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.

Trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste. Its IUPAC name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a volatile anesthetic and as an inhaled obstetrical analgesic. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

A correction fluid is an opaque, usually white fluid applied to paper to mask errors in text. Once dried, it can be handwritten or typed upon. It is typically packaged in small bottles, with lids attached to brushes that dip into the fluid. The brush applies the fluid to the paper.
Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

Tipp-Ex is a brand of correction fluid and other related products that is popular throughout Europe. It was also the name of the German company that produced the products in the Tipp-Ex line. While Tipp-Ex is a trademark name for correction products, in some countries it has become a genericised trademark: to tippex or to tippex out means to erase, either generally or with correction fluid.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.
1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.
1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. They are both colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, but soluble in organic solvents. 1,1-DCE was the precursor to the original clingwrap, Saran, for food, but this application has been phased out.
1,1,2-Trichloroethane, or 1,1,2-TCA, is an organochloride solvent with the molecular formula C2H3Cl3 and the structural formula CH2Cl—CHCl2. It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane, and a byproduct of its manufacture.
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent.
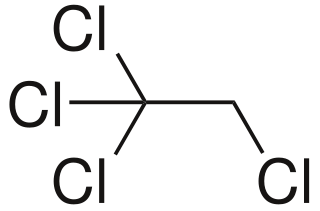
1,1,1,2-Tetrachloroethane is a chlorinated hydrocarbon. It is a colorless liquid with a sweet chloroform-like odor. It is used as a solvent and in the production of wood stains and varnishes. It is an isomer of 1,1,2,2-tetrachloroethane.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC−CClF2. This colorless, volatile liquid is a versatile solvent.

Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.
















