
2C-D is a psychedelic drug of the 2C family that is sometimes used as an entheogen. It was first synthesized in 1970 by a team from the Texas Research Institute of Mental Sciences, and its activity was subsequently investigated in humans by Alexander Shulgin. In his book PiHKAL, Shulgin lists the dosage range as being from 20 to 60 mg. Lower doses of 10 mg or less have been explored for microdosing.

3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
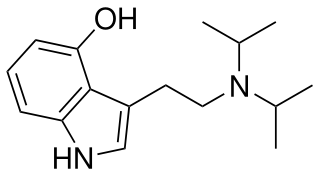
4-Hydroxy-N,N-diisopropyltryptamine is a synthetic psychedelic drug. It is a higher homologue of psilocin, 4-HO-DET, and is a positional isomer of 4-HO-DPT and has a tryptamine molecular sub-structure.

Benzylpiperazine (BZP) is a recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine. Adverse effects have been reported following its use including acute psychosis, renal toxicity and seizures. Deaths from piperazine derivatives are extremely rare, but there has been at least one death apparently due to BZP alone. Its sale is banned in several countries, including Australia, Canada, New Zealand, the United States, the Republic of Ireland, the United Kingdom, Bulgaria, Romania and other parts of Europe.

5-MeO-MiPT is a psychedelic and hallucinogenic drug, used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs 5-MeO-DiPT, DiPT, and MiPT. It is commonly used as a "substitute" for 5-MeO-DiPT because of the very similar structure and effects.
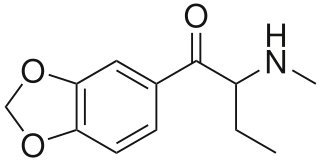
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine chemical class. It is the β-keto analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone.
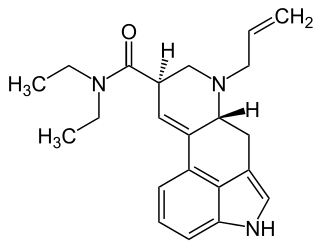
AL-LAD, also known as 6-allyl-6-nor-LSD, is a psychedelic drug and an analog of lysergic acid diethylamide (LSD). It is described by Alexander Shulgin in the book TiHKAL. It is synthesized starting from nor-LSD as a precursor, using allyl bromide as a reactant.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.

para-Methoxyphenylpiperazine is a piperazine derivative with stimulant effects which has been sold as an ingredient in "Party pills", initially in New Zealand and subsequently in other countries around the world.

para-Fluorophenylpiperazine is a piperazine derivative with mildly psychedelic and euphoriant effects. It has been sold as an ingredient in legal recreational drugs known as "Party pills", initially in New Zealand and subsequently in other countries around the world.

Methylbenzylpiperazine is a stimulant drug which is a derivative of benzylpiperazine. MBZP has been sold as an ingredient in legal recreational drugs known as "party pills", initially in New Zealand and subsequently in other countries around the world.
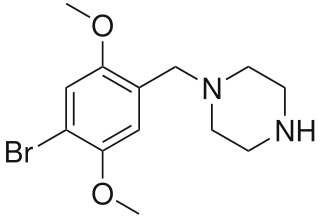
4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) is a psychoactive drug and research chemical of the piperazine chemical class which has been sold as a "designer drug". It produces stimulant effects similar to those of benzylpiperazine (BZP).
Dibenzylpiperazine (DBZP) is a piperazine derivative often found as an impurity in the recreational stimulant drug benzylpiperazine (BZP). Presence of DBZP is a marker for low quality or badly made BZP. It can be made as a reaction byproduct during BZP synthesis, either because the reaction has been run at too high a temperature, or because an excess of benzyl chloride has been used.

5-APB is an empathogenic psychoactive compound of the substituted benzofuran, substituted amphetamine and substituted phenethylamine classes. 5-APB and other compounds are sometimes informally called "Benzofury".

AB-FUBINACA is a psychoactive drug that acts as a potent agonist for the cannabinoid receptors, with Ki values of 0.9 nM at CB1 and 23.2 nM at CB2 and EC50 values of 1.8 nM at CB1 and 3.2 nM at CB2. It was originally developed by Pfizer in 2009 as an analgesic medication but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan, along with a related compound AB-PINACA, which had not previously been reported.
5-MAPB is an entactogenic designer drug similar to MDMA in its structure and effects.

1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine (3C-PEP) is a designer drug of the piperazine class of chemical substances. 3C-PEP is related to meta-cholorophenylpiperazine (mCPP) and phenethylamine that can be thought of as mCPP having a phenylethyl group attached to the nitrogen atom at its 4-position. It was first described in 1994 in a patent disclosing a series of piperazine compounds as sigma receptor ligands. Later, it was discovered to be a highly potent dopamine reuptake inhibitor.
Substituted piperazines are a class of chemical compounds based on a piperazine core. Some are used as recreational drugs and some are used in scientific research.

ortho-Methylphenylpiperazine (also known as oMPP, oMePP, 1-(2-methylphenyl)piperazine, 2-MPP, and 2-MePP) is a psychoactive designer drug of the phenylpiperazine group. It acts as a serotonin–norepinephrine–dopamine releasing agent (SNDRA), with EC50 values for induction of monoamine release of 175 nM for serotonin, 39.1 nM for norepinephrine, and 296–542 nM for dopamine. As such, it has about 4.5-fold preference for induction of norepinephrine release over serotonin, and about 7.6- to 13.9-fold preference for induction of norepinephrine release over dopamine.

N-Ethyl-2C-B is a recreational designer drug with psychedelic effects. It was first synthesised in the 1990s, and was first identified as a new psychoactive substance in Finland in 2007. It is specifically listed as an illegal drug in Finland, and controlled under analogue provisions in a number of other jurisdictions.






















