An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may be used for severe cases of gastroenteritis, especially if the patient is dehydrated.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

The 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.

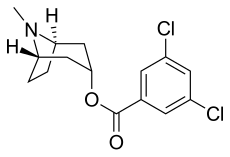
Tropisetron is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic to treat nausea and vomiting following chemotherapy, although it has been used experimentally as an analgesic in cases of fibromyalgia.
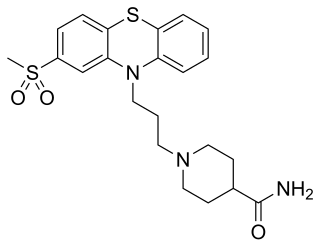
Metopimazine, sold under the brand names Vogalen and Vogalene, is an antiemetic of the phenothiazine group which is used to treat nausea and vomiting. It is marketed in Europe, Canada, and South America. As of August 2020, metopimazine has been repurposed and is additionally under development for use in the United States for the treatment of gastroparesis.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Antalarmin (CP-156,181) is a drug that acts as a CRH1 antagonist.

Zacopride is a potent antagonist at the 5-HT3 receptor and an agonist at the 5-HT4 receptor. It has anxiolytic and nootropic effects in animal models, with the (R)-(+)-enantiomer being the more active form. It also has antiemetic and pro-respiratory effects, both reducing sleep apnea and reversing opioid-induced respiratory depression in animal studies. Early animal trials have also revealed that administration of zacopride can reduce preference for and consumption of ethanol.

Zatosetron (LY-277,359) is a drug which acts as an antagonist at the 5HT3 receptor It is orally active and has a long duration of action, producing antinauseant effects but without stimulating the rate of gastrointestinal transport. It is also an effective anxiolytic in both animal studies and human trials, although with some side effects at higher doses.

Leonurine is a pseudoalkaloid that has been isolated from Leonotis leonurus, Leonotis nepetifolia, Leonurus japonicus, Leonurus cardiaca (motherwort), Leonurus sibiricus, as well as other plants of family Lamiaceae. Leonurine is easily extracted into water.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

Dazopride (AHR-5531) is an antiemetic and gastroprokinetic agent of the benzamide class which was never marketed. It acts as a 5-HT3 receptor antagonist and 5-HT4 receptor agonist. In addition to its gastrointestinal effects, dazopride facilitates learning and memory in mice.
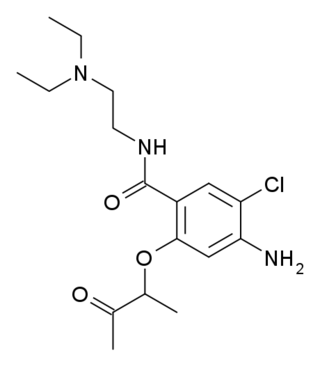
Batanopride (BMY-25,801) is an antiemetic drug of the benzamide class which acts as a selective 5-HT3 receptor antagonist. It was trialled to reduce nausea during cancer chemotherapy, but was never approved for medical use due to dose-limiting side effects including hypotension and long QT syndrome.

Piquindone (Ro 22-1319) is an atypical antipsychotic with a tricyclic structure that was developed in the 1980s but was never marketed. It acts as a selective D2 receptor antagonist, though based on its effects profile its selectivity may be considered controversial. Unlike most other D2 receptor ligands, piquindone displays Na+-dependent binding, a property it shares with tropapride, zetidoline, and metoclopramide.
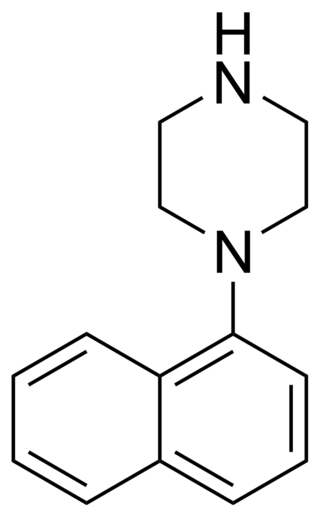
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

SKF-97,541 is a compound used in scientific research which acts primarily as a selective GABAB receptor agonist. It has sedative effects in animal studies and is widely used in research into potential treatment of various types of drug addiction.

meta-Chlorophenylbiguanide (1-(3-Chlorophenylbiguanide, m-CPBG) is an allosteric agonist and modulator of the 5-HT3 receptor and an antagonist of the α2A-adrenergic receptor. It has anxiogenic, emetic and hypothermic effects in animal studies.
Chemotherapy-induced nausea and vomiting (CINV) is a common side-effect of many cancer treatments. Nausea and vomiting are two of the most feared cancer treatment-related side effects for cancer patients and their families. In 1983, Coates et al. found that patients receiving chemotherapy ranked nausea and vomiting as the first and second most severe side effects, respectively. Up to 20% of patients receiving highly emetogenic agents in this era postponed, or even refused, potentially curative treatments. Since the 1990s, several novel classes of antiemetics have been developed and commercialized, becoming a nearly universal standard in chemotherapy regimens, and helping to better manage these symptoms in a large portion of patients. Efficient mediation of these unpleasant and sometimes debilitating symptoms results in increased quality of life for the patient, and better overall health of the patient, and, due to better patient tolerance, more effective treatment cycles.