
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom (=N−). It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Zinc chloride is an inorganic chemical compound with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates, are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.
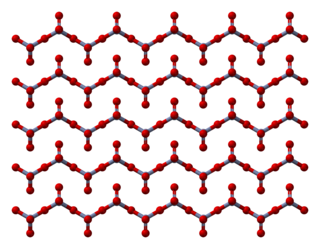
Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.

Chromium(III) picolinate is a chemical compound with the formula Cr(C5H4N )3, commonly abbreviated as CrPic3. It is a bright-red coordination compound derived from chromium(III) and picolinic acid.

o-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. OPD is a white compound although samples appear darker owing to oxidation by air. It is isomeric with m-phenylenediamine and p-phenylenediamine.
Isonicotinic acid or pyridine-4-carboxylic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid substituent at the 4-position. It is an isomer of picolinic acid and nicotinic acid, which have the carboxyl group at the 2- and 3-position respectively compared to the 4-position for isonicotinic acid.

Vanadium compounds are compounds formed by the element vanadium (V). The chemistry of vanadium is noteworthy for the accessibility of the four adjacent oxidation states 2–5, whereas the chemistry of the other group 5 elements, niobium and tantalum, are somewhat more limited to the +5 oxidation state. In aqueous solution, vanadium forms metal aquo complexes of which the colours are lilac [V(H2O)6]2+, green [V(H2O)6]3+, blue [VO(H2O)5]2+, yellow-orange oxides [VO(H2O)5]3+, the formula for which depends on pH. Vanadium(II) compounds are reducing agents, and vanadium(V) compounds are oxidizing agents. Vanadium(IV) compounds often exist as vanadyl derivatives, which contain the VO2+ center.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2N−C(=S)−S−R and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

Sodium dimethyldithiocarbamate is the organosulfur compound with the formula NaS2NN(CH3)2. It is one of the simplest organic dithiocarbamates. It is a white or pale yellow, water soluble solid. The compound is a precursor to fungicides and rubber chemicals.
2-Methylpyridine, or 2-picoline, is the compound described with formula C6H7N. 2-Picoline is a colorless liquid that has an unpleasant odor similar to pyridine. It is mainly used to make vinylpyridine and the agrichemical nitrapyrin.
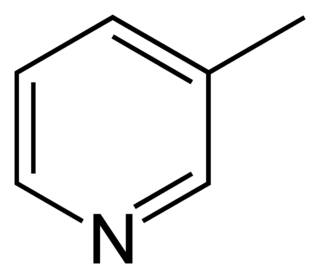
3-Methylpyridine or 3-picoline, is an organic compound with formula 3-CH3C5H4N. It is one of three positional isomers of methylpyridine, whose structures vary according to where the methyl group is attached around the pyridine ring. This colorless liquid is a precursor to pyridine derivatives that have applications in the pharmaceutical and agricultural industries. Like pyridine, 3-methylpyridine is a colorless liquid with a strong odor and is classified as a weak base.

Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peroxyacids as the oxidising agent. The compound is used infrequently as an oxidizing reagent in organic synthesis.
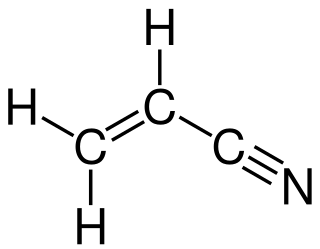
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:

Zinc picolinate (or ZnPic) is the zinc coordination complex derived from picolinic acid and zinc(II). It has the formula Zn(NC5H4CO2)2(H2O)2. The complex adopts an octahedral molecular geometry, containing two bidentate picolinate ligands (conjugate base of picolinic acid) and two aquo ligands. Additionally, two water of crystallization are present, thus the compound crystallizes with the formula Zn(NC5H4CO2)2(H2O)2·2H2O. It is a colorless solid.

Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white, hygroscopic solid. It forms a hydrate.

5-Ethyl-2-methylpyridine is an organic compound with the formula (C2H5)(CH3)C5H3N. One of several isomeric pyridines with this formula, this derivative is of interest because it is efficiently prepared from simple reagents and it is a convenient precursor to nicotinic acid, a form of vitamin B3. 5-Ethyl-2-methylpyridine is a colorless liquid.



















