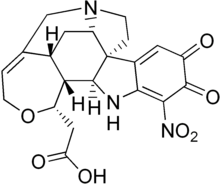
Strychnine is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia. While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant and performance-enhancing drug. The most common source is from the seeds of the Strychnos nux-vomica tree.

Jane Elizabeth Lathrop Stanford was an American philanthropist and co-founder of Stanford University in 1885, along with her husband, Leland Stanford, in memory of their only child, Leland Stanford Jr., who died of typhoid fever at age 15 in 1884. After her husband's death in 1893, she funded and operated the university almost single-handedly until her unsolved murder by strychnine poisoning in 1905.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
The year 1819 in science and technology involved some significant events, listed below.

Strychnos nux-vomica, the strychnine tree, also known as nux vomica, poison fruit, semen strychnos, and quaker buttons, is a deciduous tree native to India and to southeast Asia. It is a medium-sized tree in the family Loganiaceae that grows in open habitats. Its leaves are ovate and 5–9 centimetres (2–3.5 in) in size. It is known for being the natural source of the extremely poisonous compound strychnine.

Brucine, is an alkaloid closely related to strychnine, most commonly found in the Strychnos nux-vomica tree. Brucine poisoning is rare, since it is usually ingested with strychnine, and strychnine is more toxic than brucine. In synthetic chemistry, it can be used as a tool for stereospecific chemical syntheses.

The glycine receptor is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory receptors in the central nervous system and has important roles in a variety of physiological processes, especially in mediating inhibitory neurotransmission in the spinal cord and brainstem.

Dextromethorphan (DXM) is a cough suppressant in over-the-counter cold and cough medicines. It affects NMDA, glutamate-1, and sigma-1 receptors in the brain, all of which have been implicated in the pathophysiology of depression. In 2022, the FDA approved a formulation of it combined with bupropion named Auvelity to serve as a rapid acting antidepressant in patients with major depressive disorder. It is sold in syrup, instant release tablet, extended release tablet, spray, and lozenge forms.

The Mechanic is a 1972 American action thriller film directed by Michael Winner from a screenplay by Lewis John Carlino. It stars Charles Bronson, in his first collaboration with Winner, Jan-Michael Vincent, Keenan Wynn, and Jill Ireland.
Bursinel is a municipality in the district of Nyon in the canton of Vaud in Switzerland.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.
A bitterant is a chemical that is added to a product to make it smell or taste bitter. Bitterants are commonly used as aversive agents to discourage the inhalation or ingestion of toxic substances.

Strychnos ignatii is a tree in the family Loganiaceae, native to the Philippines, particularly in Catbalogan and parts of China. The plant was first described by the Moravian (Czech) Jesuit working in the Philippines, brother Georg Kamel who named its seeds "the beans of St. Ignatius", in honour of the founder of his religious order.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.
George Roger Clemo FRS was a British organic chemist.
A glycine receptor antagonist is a drug which acts as an antagonist of the glycine receptor.
2-Methylbutanoic acid, also known as 2-methylbutyric acid is a branched-chain alkyl carboxylic acid with the chemical formula CH3CH2CH(CH3)CO2H, classified as a short-chain fatty acid. It exists in two enantiomeric forms, (R)- and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic occurs in many fruits such as apples and apricots, as well as in the scent of the orchid Luisia curtisii.
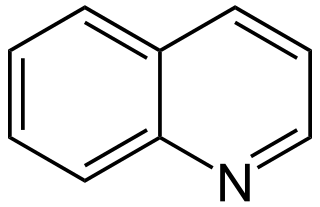
Quinoline alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from quinoline. Some quinoline alkaloids show antiseptic, convulsive or antineoplastic effects.

Chiral thin-layer chromatography is a variant of liquid chromatography that is employed for the separation of enantiomers. It is necessary to use either