
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Buspirone, sold under the brand name Buspar, among others, is a medication primarily used to treat anxiety disorders, particularly generalized anxiety disorder. Benefits support its short-term use. It is taken orally, and takes two to six weeks to be fully effective.

Pindolol, sold under the brand name Visken among others, is a nonselective beta blocker which is used in the treatment of hypertension. It is also an antagonist of the serotonin 5-HT1A receptor, preferentially blocking inhibitory 5-HT1A autoreceptors, and has been researched as an add-on therapy to selective serotonin reuptake inhibitors (SSRIs) in the treatment of depression.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.

Etoperidone, associated with several brand names, is an atypical antidepressant which was developed in the 1970s and either is no longer marketed or was never marketed. It is a phenylpiperazine related to trazodone and nefazodone in chemical structure and is a serotonin antagonist and reuptake inhibitor (SARI) similarly to them.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
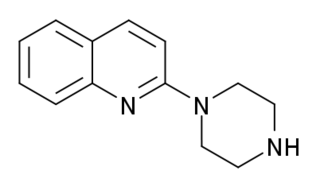
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use.

CP-94253 is a drug which acts as a potent and selective serotonin 5-HT1B receptor agonist, with approximately 25x and 40x selectivity over the closely related 5-HT1D and 5-HT1A receptors. It has a range of behavioral effects, based on animal testing. The effects include the following: promoting wakefulness by increasing dopamine release in the brain; reducing food intake and promoting satiety; enhancing the reinforcing effects of cocaine; and possible antidepressant effects. A recent study found that "Regardless of sex, CP94253 decreased cocaine intake after abstinence and during resumption of SA [self-administration] and decreased cue reactivity" suggesting that agonism of the inhibitory 5-HT2B receptors may diminish the cognitive reward of cocaine usage and increased use of the drug without a period of abstinence may be a product of test subjects trying to achieve a previously rewarding experience through larger dosages of cocaine.
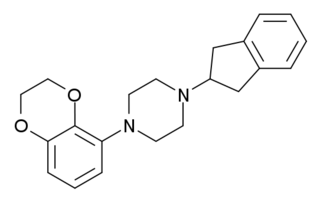
S-15535 is a phenylpiperazine drug which is a potent and highly selective 5-HT1A receptor ligand that acts as an agonist and antagonist at the presynaptic and postsynaptic 5-HT1A receptors, respectively. It has anxiolytic properties.

Ricasetron (BRL-46470) is a drug which acts as a selective antagonist at the serotonin 5-HT3 receptor. It has antiemetic effects as with other 5-HT3 antagonists, and also has anxiolytic effects significantly stronger than other related drugs, and with less side effects than benzodiazepine anxiolytics. However, it has never been developed for medical use.

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

Adatanserin is a mixed 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2C receptor antagonist. It was under development by Wyeth as an antidepressant but was ultimately not pursued.
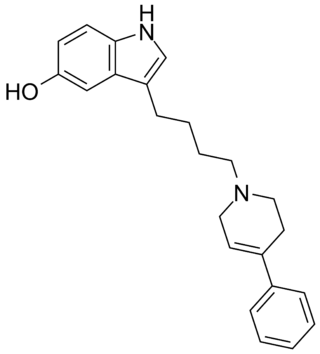
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor. Osemozotan has antidepressant, anxiolytic, antiobsessional, serenic, and analgesic effects in animal studies, and is used to investigate the role of 5-HT1A receptors in modulating the release of dopamine and serotonin in the brain, and their involvement in addiction to abused stimulants such as cocaine and methamphetamine.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

CSP-2503 is a potent and selective 5-HT1A receptor agonist, 5-HT2A receptor antagonist, and 5-HT3 receptor antagonist of the naphthylpiperazine class. First synthesized in 2003, it was designed based on computational models and QSAR studies. In rat studies, CSP-2503 has demonstrated anxiolytic effects, and thus has been suggested as a treatment for anxiety in humans with a multimodal mechanism of action.

SB-243213 is a research chemical which acts as a selective inverse agonist for the 5HT2C receptor and has anxiolytic effects. It has better than 100x selectivity for 5-HT2C over all other receptor subtypes tested, and a longer duration of action compared to older 5-HT2C antagonist ligands.





















