
PiHKAL: A Chemical Love Story is a book by Dr. Alexander Shulgin and Ann Shulgin, published in 1991. The subject of the work is psychoactive phenethylamine chemical derivatives, notably those that act as psychedelics and/or empathogen-entactogens. The main title, PiHKAL, is an acronym that stands for "Phenethylamines I Have Known and Loved."

2C-I (2,5-Dimethoxy-4-iodophenethylamine) is a phenethylamine of the 2C family with psychedelic properties. It was first synthesized by Alexander Shulgin and described in his 1991 book PiHKAL. The drug has been used recreationally as psychedelic and other reported effects and was sometimes confused with the more potent chemical cousin 25I-NBOMe, nicknamed "Smiles," in the media.
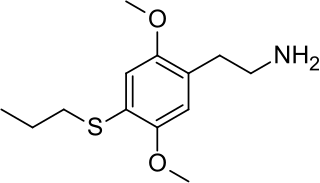
2C-T-7 is a psychedelic phenethylamine of the 2C family. In his book PiHKAL: A Chemical Love Story, Alexander Shulgin lists the dosage range as 10–30 mg. 2C-T-7 is generally taken orally, and produces psychedelic and entactogenic effects that last 8 to 15 hours. Up until Operation Web Tryp and three deaths, two of which involved the use of other drugs in addition to 2C-T-7, and one which involved an excessive insufflated dose, 2C-T-7 was sold commercially in Dutch and Japanese smartshops and online. It is known on the streets as Blue Mystic or 7th Heaven. There has been little real research done on this chemical other than Shulgin's comments in PiHKAL and a few small animal studies mostly aimed at detecting metabolites.
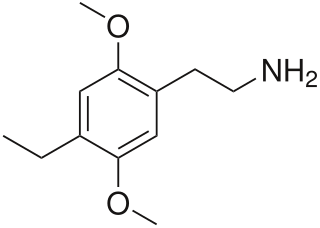
2C-E is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and documented in his book PiHKAL. Like the other substances in its family, it produces sensory and cognitive effects in its physical reactions with living organisms.

2C-C is a psychedelic drug of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen. In his book PiHKAL , Shulgin lists the dosage range as 20–40 mg. 2C-C is usually taken orally, but may also be insufflated. 2C-C is schedule I of section 202(c) of the Controlled Substances Act in the United States, signed into law as of July, 2012 under the Food and Drug Administration Safety and Innovation Act.

2C-D is a psychedelic drug of the 2C family that is sometimes used as an entheogen. It was first synthesized in 1970 by a team from the Texas Research Institute of Mental Sciences, and its activity was subsequently investigated in humans by Alexander Shulgin. In his book PiHKAL, Shulgin lists the dosage range as being from 20 to 60 mg. Lower doses of 10 mg or less have been explored for microdosing.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.

Dimethoxybromoamphetamine (DOB), also known as brolamfetamine (INN) and bromo-DMA, is a psychedelic drug and substituted amphetamine of the phenethylamine class of compounds. DOB was first synthesized by Alexander Shulgin in 1967. Its synthesis and effects are documented in Shulgin's book PiHKAL: A Chemical Love Story.

2C-T-8 is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen.

2C-N (2,5-dimethoxy-4-nitrophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin.
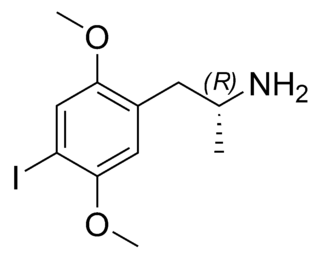
2,5-Dimethoxy-4-iodoamphetamine (DOI) is a psychedelic drug and a substituted amphetamine. Unlike many other substituted amphetamines, however, it is not primarily a stimulant. DOI has a stereocenter and R-(−)-DOI is the more active stereoisomer. In neuroscience research, [125I]-R-(−)-DOI is used as a radioligand and indicator of the presence of 5-HT2A serotonin receptors. DOI's effects have been compared to LSD, although there are differences that experienced users can distinguish. Besides the longer duration, the trip tends to be more energetic than an LSD trip, with more body load and a different subjective visual experience. The after effects include residual stimulation and difficulty sleeping, which, depending on the dose, may persist for days. While rare, it is sometimes sold as a substitute for LSD, or even sold falsely as LSD, which may be dangerous because DOI does not have the same established safety profile as LSD.

2C-T-4 (2,5-dimethoxy-4-isopropylthiophenethylamine) is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin and is used as entheogenic recreational drug.

2C-G is a psychedelic phenethylamine of the 2C-series. First synthesized by Alexander Shulgin, it is sometimes used as an entheogen. It has structural and pharmacodynamic properties similar to 2C-D and Ganesha. Like many of the phenethylamines in PiHKAL, 2C-G and its homologs have only been taken by Shulgin and a small test group, making it difficult to ensure completeness when describing effects.

2C-T is a psychedelic and hallucinogenic drug of the 2C family. It is used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs mescaline and 2C-T-2.
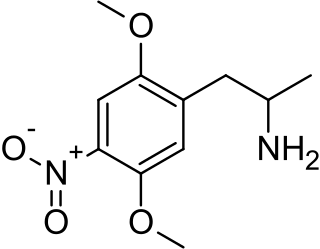
2,5-Dimethoxy-4-nitroamphetamine (DON) is a psychedelic drug and amphetamine. It is an analog of DOM and DOB. It is also closely related to 2C-N.

2,5-Dimethoxy-4-ethylamphetamine is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL.
Ganesha (2,5-dimethoxy-3,4-dimethylamphetamine) is a lesser-known psychedelic drug. It is also a substituted amphetamine. It was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 24–32 mg. The drug is usually taken orally, although other routes such as rectally may also be used. Ganesha is synthesized from 2,5-dimethoxy-3,4-dimethylbenzaldehyde. Ganesha is the amphetamine analog of 2C-G. It is a particularly long lasting drug, with the duration listed in PiHKAL as being 18–24 hours, which might make it undesirable to some users. It is named after the Hindu deity, Ganesha. Very little is known about the dangers or toxicity of ganesha. Effects of ganesha include:

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin, who named it after the first letter of the Hebrew alphabet. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg, with effects typically lasting for 6 to 8 hours.

2C-T-15 or 2,5-dimethoxy-4-(β-cyclopropylthio)phenethylamine is a psychedelic phenethylamine of the 2C family. It was presumably first synthesized by Alexander Shulgin and reported in his book PiHKAL .

2,5-Dimethoxy-4-fluoroamphetamine (DOF) is a psychedelic drug of the phenethylamine and amphetamine classes. Alexander Shulgin briefly describes DOF in his book PiHKAL:
Animal studies that have compared DOF to the highly potent DOI and DOB imply that the human activity will be some four to six times less than these two heavier halide analogues.




















