
APICA is an indole based drug that acts as a potent agonist for the cannabinoid receptors.

AB-FUBINACA (AMB-FUBINACA) is a psychoactive drug that acts as a potent agonist for the cannabinoid receptors, with Ki values of 0.9 nM at CB1 and 23.2 nM at CB2 and EC50 values of 1.8 nM at CB1 and 3.2 nM at CB2. It was originally developed by Pfizer in 2009 as an analgesic medication but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan, along with a related compound AB-PINACA, which had not previously been reported.

AB-PINACA is a compound that was first identified as a component of synthetic cannabis products in Japan in 2012.

ADB-FUBINACA (ADMB-FUBINACA) is a designer drug identified in synthetic cannabis blends in Japan in 2013. In 2018, it was the third-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

AB-CHMINACA is an indazole-based synthetic cannabinoid. It is a potent agonist of the CB1 receptor (Ki = 0.78 nM) and CB2 receptor (Ki = 0.45 nM) and fully substitutes for Δ9-THC in rat discrimination studies, while being 16x more potent. Continuing the trend seen in other cannabinoids of this generation, such as AB-FUBINACA and AB-PINACA, it contains a valine amino acid amide residue as part of its structure, where older cannabinoids contained a naphthyl or adamantane residue.
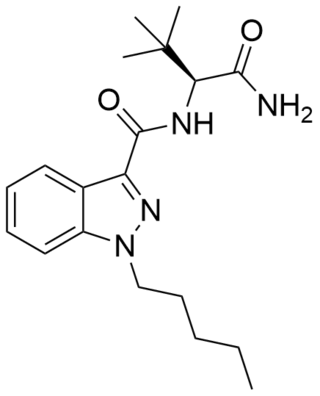
ADB-PINACA is a cannabinoid designer drug that is an ingredient in some synthetic cannabis products. It is a potent agonist of the CB1 receptor and CB2 receptor with EC50 values of 0.52 nM and 0.88 nM respectively. Like MDMB-FUBINACA, this compound incorporates the unnatural amino acid tert-leucine.

5F-ADB (also known as MDMB-5F-PINACA and 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug. 5F-ADB is a potent agonist of the CB1 receptor, though it is unclear whether it is selective for this target. 5F-ADB was first identified in November 2014 from post-mortem samples taken from an individual who had died after using a product containing this substance. Subsequent testing identified 5F-ADB to have been present in a total of ten people who had died from unexplained drug overdoses in Japan between September 2014 and December 2014. 5F-ADB is believed to be extremely potent based on the very low levels detected in tissue samples, and appears to be significantly more toxic than earlier synthetic cannabinoid drugs that had previously been sold.
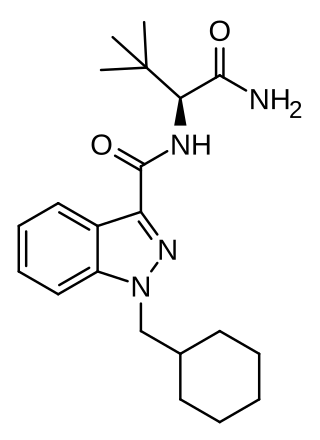
ADB-CHMINACA (also known as ADMB-CHMINACA and MAB-CHMINACA) is an indazole-based synthetic cannabinoid. It is a potent agonist of the CB1 receptor with a binding affinity of Ki = 0.289 nM and was originally developed by Pfizer in 2009 as an analgesic medication. It was identified in cannabinoid blends in Japan in early 2015.
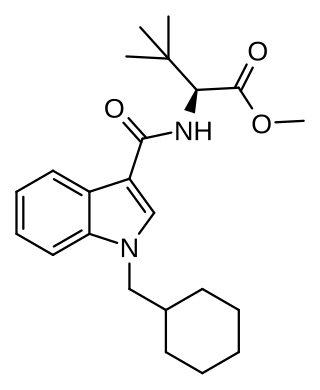
'MDMB-CHMICAa' is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug. While MDMB-CHMICA was initially sold under the name "MMB-CHMINACA", the compound corresponding to this code name (i.e. the isopropyl instead of t-butyl analogue of MDMB-CHMINACA) has been identified on the designer drug market in 2015 as AMB-CHMINACA.

5F-AB-PINACA is an indazole-based synthetic cannabinoid that is derived from a series of compounds originally developed by Pfizer in 2009 as an analgesic medication, and has been sold online as a designer drug.

5F-APINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. Structurally it closely resembles cannabinoid compounds from patent WO 2003/035005 but with a 5-fluoropentyl chain on the indazole 1-position, and 5F-APINACA falls within the claims of this patent, as despite not being disclosed as an example, it is very similar to the corresponding pentanenitrile and 4-chlorobutyl compounds which are claimed as examples 3 and 4.
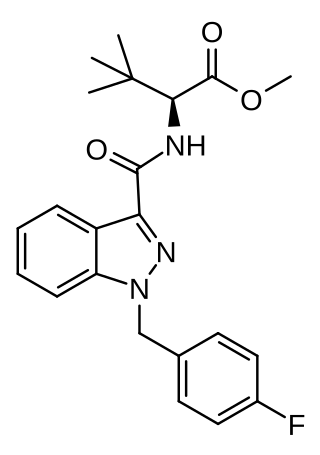
MDMB-FUBINACA (also known as MDMB(N)-Bz-F and FUB-MDMB) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with Ki values of 1.14 nM at CB1 and 0.1228 nM at CB2 and EC50 values of 0.2668 nM at CB1 and 0.1411 nM at CB2, and has been sold online as a designer drug. Its benzyl analogue (instead of 4-fluorobenzyl) has been reported to be a potent agonist for the CB1 receptor (Ki = 0.14 nM, EC50 = 2.42 nM). The structure of MDMB-FUBINACA contains the amino acid, 3-methylvaline or tert-leucine methyl ester.

PX-2 is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. It contains a phenylalanine amino acid amide as part of its structure.
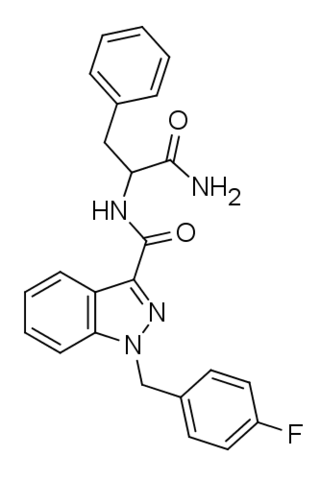
APP-FUBINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. Pharmacological testing showed APP-FUBINACA to have only moderate affinity for the CB1 receptor, with a Ki of 708 nM, while its EC50 was not tested. It contains a phenylalanine amino acid residue in its structure.

MMB-2201 is a potent indole-3-carboxamide based synthetic cannabinoid, which has been sold as a designer drug and as an active ingredient in synthetic cannabis blends. It was first reported in Russia and Belarus in January 2014, but has since been sold in a number of other countries. In the United States, MMB-2201 was identified in Drug Enforcement Administration drug seizures for the first time in 2018.

5F-ADB-PINACA is a cannabinoid designer drug that is an ingredient in some synthetic cannabis products. It is a potent agonist of the CB1 receptor and CB2 receptor with EC50 values of 0.24 nM and 2.1 nM respectively.

AMB-FUBINACA (also known as FUB-AMB and MMB-FUBINACA) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with Ki values of 10.04 nM at CB1 and 0.786 nM at CB2 and EC50 values of 0.5433 nM at CB1 and 0.1278 nM at CB2, and has been sold online as a designer drug. It was originally developed by Pfizer which described the compound in a patent in 2009, but was later abandoned and never tested on humans. AMB-FUBINACA was the most common synthetic cannabinoid identified in drug seizures by the Drug Enforcement Administration in 2017 and the first half of 2018.

AMB-CHMINACA or MMB-CHMINACA (also known as MA-CHMINACA) is an indazole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug.
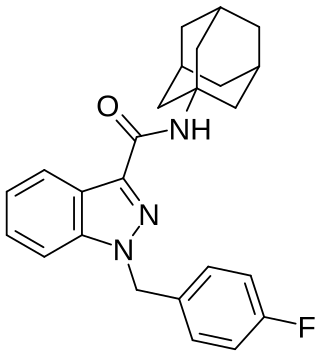
FUB-APINACA (also known as A-FUBINACA according to the EMCCDA framework for naming synthetic cannabinoids and FUB-AKB48) is an indazole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is an analog of APINACA and 5F-APINACA where the pentyl chain has been replaced with fluorobenzyl.
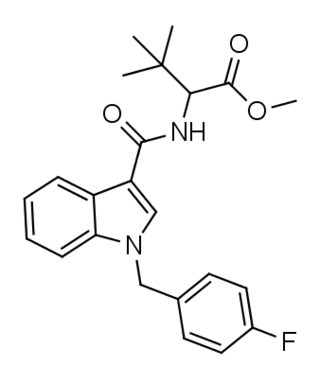
MDMB-FUBICA is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug.




















