
JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

AMG-3 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a dithiolane group on the 3-position side chain. AMG-3 is a potent agonist at both CB1 and CB2 receptors with a Ki of 0.32 nM at CB1 and 0.52 nM at CB2, and its particularly high binding affinity has led to it being used as a template for further structural development of novel cannabinoid drugs. It has sedative and analgesic effects, with analgesia lasting for up to 36 hours after administration.
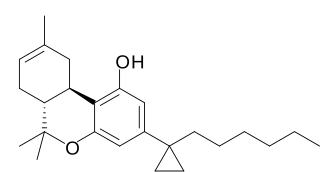
AMG-41 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a cyclopropyl group on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.44 nM at CB1 vs 0.86 nM at CB2.

AM-4030 is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-((E)-3-hydroxyprop-1-enyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-4030 represents a hybrid structure between the classical and nonclassical cannabinoid families, with the 6-hydroxyalkyl chain rigidified with a double bond with defined stereochemistry. This gives AM-4030 a greater degree of selectivity, so while it is still a potent agonist at both CB1 and CB2, it is reasonably selective for CB1, with a Ki of 0.7nM at CB1 and 8.6nM at CB2, a selectivity of around 12x. Resolution of the enantiomers of AM-4030 yields an even more potent compound, although with less selectivity, with the (−) enantiomer AM-4030a having a Ki of 0.6nM at CB1 and 1.1nM at CB2.

GW-405,833 (L-768,242) is a drug that acts as a potent and selective partial agonist for the cannabinoid receptor subtype CB2, with an EC50 of 0.65 nM and selectivity of around 1200x for CB2 over CB1 receptors. Animal studies have shown it to possess antiinflammatory and anti-hyperalgesic effects at low doses, followed by ataxia and analgesic effects when the dose is increased. Selective CB2 agonist drugs such as GW-405,833 are hoped to be particularly useful in the treatment of allodynia and neuropathic pain for which current treatment options are often inadequate.

A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2Ki of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects.

SER-601 (COR-167) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist, based on a quinolone-3-carboxylic acid core structure, with 190 times selectivity for CB2 over the related CB1 receptor. It has analgesic effects in animal studies, as well as neuroprotective effects, but without a "cannabis high" due to its low affinity for CB1. A number of related compounds are known, almost all of which have high selectivity for CB2.
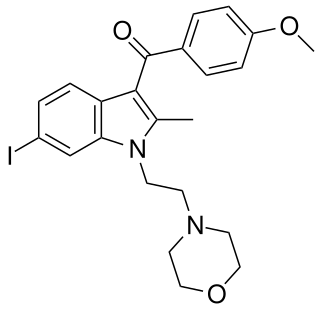
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

Org 28312 is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, but did not proceed to human trials, with the related compound Org 28611 chosen instead due to its better penetration into the brain. The structure-activity relationships of these compounds have subsequently been investigated further leading to the development of a number of more potent analogues, derived by cyclisation around the indole or piperazine rings.
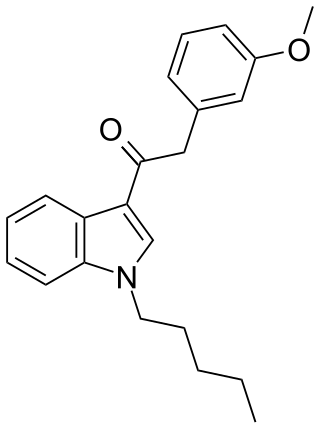
JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more common drug JWH-250, though it is slightly less potent with a Ki of 17 nM at CB1, compared to 11 nM for JWH-250. Because of their identical molecular weight and similar fragmentation patterns, JWH-302 and JWH-250 can be very difficult to distinguish by GC-MS testing.

AZ-11713908 is a drug developed by AstraZeneca which is a peripherally selective cannabinoid agonist, acting as a potent agonist at the CB1 receptor and a partial agonist at CB2. It has poor blood–brain barrier penetration, and so while it is an effective analgesic in animal tests, it produces only peripheral effects at low doses, with much weaker symptoms of central effects compared to other cannabinoid drugs such as WIN 55,212-2. Many related benzimidazole-derived cannabinoid ligands are known.
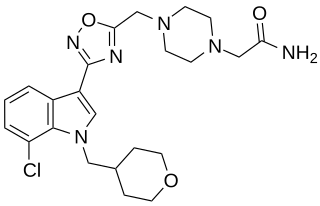
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

S-444,823 is a drug developed by Shionogi which is a cannabinoid agonist. It was developed as an antipruritic, and has moderate selectivity for the CB2 subtype, having a CB2 affinity of 18nM, and 32x selectivity over the CB1 receptor. In animal studies it showed analgesic effects and strongly reduced itching responses, but without producing side effects such as sedation and catalepsy that are seen with centrally acting CB1 agonists.

BIM-018 is a synthetic cannabinoid that is the benzimidazole analog of JWH-018. It is presumed to be a potent agonist of the CB2 receptor and has been sold online as a designer drug.

QMPSB is an arylsulfonamide-based synthetic cannabinoid that has been sold as a designer drug.

MCHB-1 is a benzimidazole derived drug which was researched as an analgesic but never developed for medical use. It acts as a potent agonist of the CB2 receptor, with an EC50 of 0.52nM at CB2, and ~30x selectivity over CB1 (Ki of 110nM at CB1 vs 3.7nM at CB2). It has been sold online as a designer drug, first being identified in Germany in December 2013.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
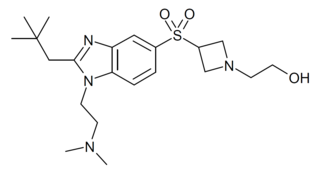
RQ-00202730 is a benzimidazole derived drug that acts as a potent and highly selective agonist for the CB2 cannabinoid receptor, with a Ki value of 19nM at CB2 and more than 4000x selectivity over CB1, though it also shows some activity as an antagonist of the unrelated 5-HT2B serotonin receptor. It has analgesic and antiinflammatory effects in animal studies, and was developed for the treatment of irritable bowel syndrome, but was ultimately discontinued from development following disappointing results in Phase II clinical trials.

ADB-FUBHQUCA is a synthetic cannabinoid receptor agonist that has been sold as a designer drug, first reported in 2022. It is related to the previously reported compound ADB-FUBICA but with the central indole ring system expanded to a 1,4-dihydroquinoline structure. This breaks the aromaticity of the ring system, and ADB-FUBHQUCA is relatively low in potency compared to related compounds where the aromatic core is retained.



















