
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.
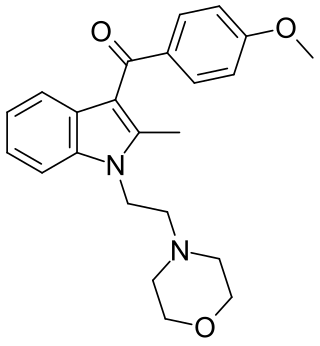
Pravadoline (WIN 48,098) is an anti-inflammatory and analgesic drug with an IC50 of 4.9 μM and a Ki of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX).
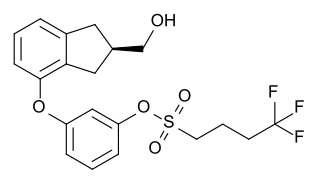
Originally synthesized by chemist Wayne E. Kenney, BAY 38-7271 (KN 38-7271) is a drug which is a cannabinoid receptor agonist developed by Bayer AG. It has analgesic and neuroprotective effects and is used in scientific research, with proposed uses in the treatment of traumatic brain injury. It is a full agonist with around the same potency as CP 55,940 in animal studies, and has fairly high affinity for both CB1 and CB2 receptors, with Ki values of 2.91nM at CB1 and 4.24nM at CB2. It has been licensed to KeyNeurotek Pharmaceuticals for clinical development, and was in Phase II trials in 2008 but its development appears to have stopped.
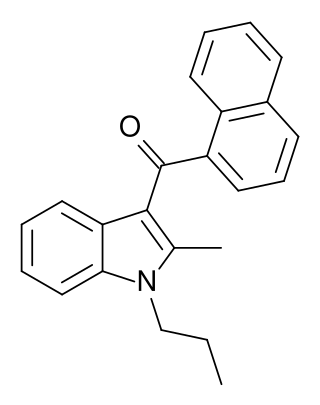
JWH-015 is a chemical from the naphthoylindole family that acts as a subtype-selective cannabinoid agonist. Its affinity for CB2 receptors is 13.8 nM, while its affinity for CB1 is 383 nM, meaning that it binds almost 28 times more strongly to CB2 than to CB1. However, it still displays some CB1 activity, and in some model systems can be very potent and efficacious at activating CB1 receptors, and therefore it is not as selective as newer drugs such as JWH-133. It has been shown to possess immunomodulatory effects, and CB2 agonists may be useful in the treatment of pain and inflammation. It was discovered and named after John W. Huffman.

JWH-030 is a research chemical which is a cannabinoid receptor agonist. It has analgesic effects and is used in scientific research. It is a partial agonist at CB1 receptors, with a Ki of 87 nM, making it roughly half the potency of THC. It was discovered and named after John W. Huffman.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.
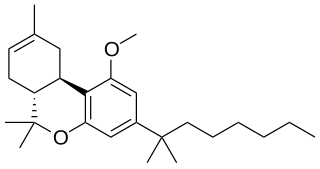
L-759,633 is an analgesic drug that is a cannabinoid agonist. It is a fairly selective agonist for the CB2 receptor, with selectivity of 163x for CB2 over CB1.

L-759,656 is an analgesic drug that is a cannabinoid agonist. It is a highly selective agonist for the CB2 receptor, with selectivity of 414x for CB2 over CB1, although it is still not as selective as newer agents such as HU-308.

JTE-907 is a drug used in scientific research that acts as a selective CB2 inverse agonist. It has antiinflammatory effects in animal studies, thought to be mediated by an interaction between the CB2 receptor and IgE.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of N-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the N-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other N-alkyl substituents were found to be active by Huffman's team including the n-butyl, n-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, with an optimum binding of 9.00 nM at CB1 and 2.94 nM at CB2, and JWH-007 displayed optimum binding of 9.50 nM at CB1 and 2.94 nM at CB2.
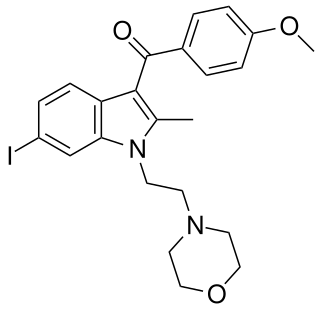
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.
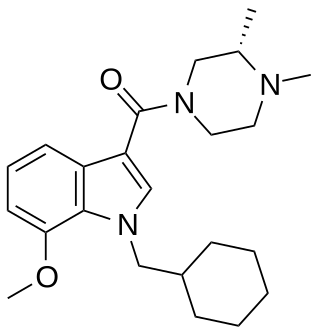
Org 28611 (SCH-900,111) is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, and while it achieved this aim and has progressed as far as Phase II clinical trials in humans as both a sedative and an analgesic, results against the comparison drugs (midazolam and morphine respectively) were not particularly favourable in initial testing.

AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a Ki of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (R) enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.
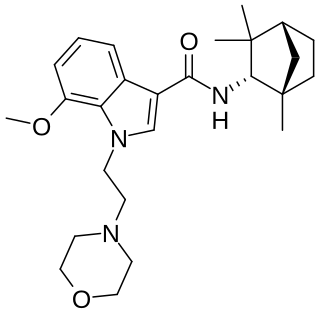
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

MDA-19 (also known as BZO-HEXOXIZID) is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with reasonable selectivity over the psychoactive CB1 receptor, though with some variation between species. In animal studies it was effective for the treatment of neuropathic pain, but did not affect rat locomotor activity in that specific study. The pharmacology of MDA-19 in rat cannabinoid receptors have been demonstrated to function differently than human cannabinoid receptors with MDA-19 binding to human CB1 receptors 6.9× higher than rat CB1 receptors.
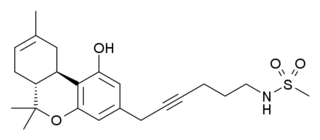
O-2050 is a drug that is a classical cannabinoid derivative, which acts as an antagonist for the CB1 receptor. This gives it an advantage in research over many commonly used cannabinoid antagonists, such as rimonabant, which at higher doses act as inverse agonists at CB1 as well as showing off-target effects. However, while O-2050 acts as a silent antagonist in vitro, some tests in vivo have suggested it may show agonist activity under certain circumstances.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.


















