
Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes. THCV was studied by Roger Adams as early as 1942.

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activate the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has moderate affinity for both CB1 and CB2 receptors, with Ki values of 8.36 nM at CB1 and 7.95 nM at CB2

JWH-030 is a research chemical which is a cannabinoid receptor agonist. It has analgesic effects and is used in scientific research. It is a partial agonist at CB1 receptors, with a Ki of 87 nM, making it roughly half the potency of THC. It was discovered and named after John W. Huffman.

NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of 350fM (i.e. 0.00035nM) and a selectivity of over 60,000x for CB1 over CB2. Independently, two other groups have described only modest nanomolar CB1 affinity for this compound (125nM and 18.4nM). Also unlike rimonabant, NESS-0327 does not appear to act as an inverse agonist at higher doses, instead being a purely neutral antagonist which blocks the CB1 receptor but does not produce any physiological effect of its own.

LY-320,135 is a drug used in scientific research which acts as a selective antagonist of the cannabinoid receptor CB1. It was developed by Eli Lilly and Company in the 1990s.

AMG-1 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC with a rigidified and extended 3-position side chain. AMG-1 is a potent agonist at both CB1 and CB2 with moderate selectivity for CB1, with a Ki of 0.6 nM at CB1 vs 3.1 nM at CB2.

O-823 is a drug which is a cannabinoid derivative that is used in scientific research. It is described as a mixed agonist/antagonist at the cannabinoid receptor CB1, meaning that it acts as an antagonist when co-administered alongside a more potent CB1 agonist, but exhibits weak partial agonist effects when administered by itself.
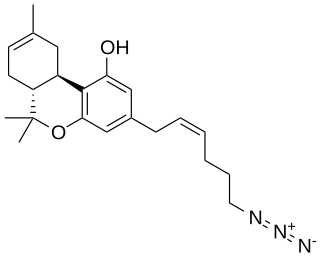
O-1238 is a drug which is a cannabinoid derivative that is used in scientific research. It is a partial agonist at the cannabinoid receptor CB1, producing a maximal stimulation of 58.3% with a Ki of 8.45 nM.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

Org 27569 is a drug which acts as a potent and selective negative allosteric modulator of the cannabinoid CB1 receptor. Studies in vitro suggest that it binds to a regulatory site on the CB1 receptor target, causing a conformational change that increases the binding affinity of CB1 agonists such as CP 55,940, while decreasing the binding affinity of CB1 antagonists or inverse agonists such as rimonabant. However while Org 27569 increases the ability of CB1 agonists to bind to the receptor, it decreases their efficacy at stimulating second messenger signalling once bound, and so in practice behaves as an insurmountable antagonist of CB1 receptor function.

O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a Ki of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes.

O-1269 is a drug that is a diarylpyrazole derivative, related to potent cannabinoid antagonist drugs such as rimonabant and surinabant. However O-1269 and several related drugs were unexpectedly found to act as full or partial agonists at the cannabinoid receptors rather than antagonists, and so produce the usual effects expected of cannabinoid agonists in animal tests, such as sedation and analgesic effects. The N-heptyl homolog O-1270 and the N-propyl homolog O-1399 also act as cannabinoid agonists with similar potency in vivo, despite weaker binding affinity at cannabinoid receptors compared to the pentyl homolog O-1269. Agonist-like and atypical cannabinoid activity has also been observed with a number of related compounds.
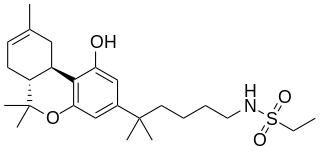
O-2113 is a drug that is a classical cannabinoid derivative, which acts as a potent agonist for cannabinoid receptors, producing sedation, hypothermia and analgesia in animal studies.

AM-6545 is a drug which acts as a peripherally selective silent antagonist for the CB1 receptor, and was developed for the treatment of obesity. Other cannabinoid antagonists such as rimonabant have been marketed for this application, but have subsequently been withdrawn from sale because of centrally mediated side effects such as depression and nausea. Because AM-6545 does not cross the blood–brain barrier to any significant extent, it does not produce these kinds of side effects, but has still been shown to effectively reduce appetite and food consumption in animal studies.
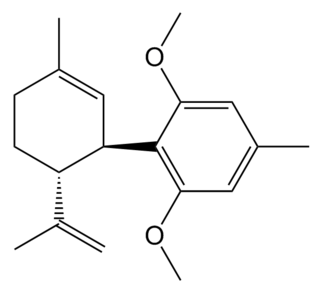
O-1918 is a synthetic compound related to cannabidiol, which is an antagonist at two former orphan receptors GPR18 and GPR55, that appear to be related to the cannabinoid receptors. O-1918 is used in the study of these receptors, which have been found to be targets for a number of endogenous and synthetic cannabinoid compounds, and are thought to be responsible for most of the non-CB1, non-CB2 mediated effects that have become evident in the course of cannabinoid research.

O-1656 is a cannabinoid agonist which was invented by Billy R Martin and Raj K Razdan at Organix Inc in 2002. It is moderately selective for the CB2 receptor with a CB1 receptor affinity of 18 nM and a CB2 receptor affinity of 2 nM. Since it has a cycloheptyl ring attached to the phenol core, it falls outside the definition of a "cyclohexylphenol derivative", but may still be controlled by generic legislation in some jurisdictions.

















