The melanocortins are a family of neuropeptide hormones which are the ligands of the melanocortin receptors. The melanocortin system consists of melanocortin receptors, ligands, and accessory proteins. The genes of the melanocortin system are found in chordates. Melanocortins were originally named so because their earliest known function was in melanogenesis. It is now known that the melanocortin system regulates diverse functions throughout the body, including inflammatory response, fibrosis, melanogenesis, steroidogenesis, energy homeostasis, sexual function, and exocrine gland function.
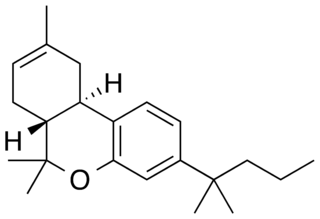
JWH-133(Dimethylbutyl-deoxy-Delta-8-THC) is a potent selective CB2 receptor agonist with a Ki of 3.4nM and selectivity of around 200x for CB2 over CB1 receptors. It was discovered by and named after, John W. Huffman.

The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.

GW-405,833 (L-768,242) is a drug that acts as a potent and selective partial agonist for the cannabinoid receptor subtype CB2, with an EC50 of 0.65 nM and selectivity of around 1200x for CB2 over CB1 receptors. Animal studies have shown it to possess antiinflammatory and anti-hyperalgesic effects at low doses, followed by ataxia and analgesic effects when the dose is increased. Selective CB2 agonist drugs such as GW-405,833 are hoped to be particularly useful in the treatment of allodynia and neuropathic pain for which current treatment options are often inadequate.
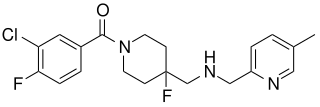
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
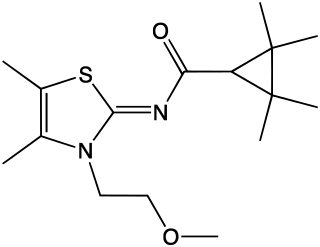
A-836,339 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist. It is selective for CB2, with Ki values of 0.64 nM at CB2 vs 270 nM at the psychoactive CB1 receptor, but while it exhibits selective analgesic, anti-inflammatory and anti-hyperalgesic effects at low doses, its high efficacy at both targets results in typical cannabis-like effects appearing at higher doses, despite its low binding affinity for CB1. In 2012 A-836,339 was detected via X-ray crystallography in a "dubious product" sold in Japan, though the product was described as a white powder, not herbal incense, it was suggested to be for human consumption.

A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2Ki of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects.

AZ-11713908 is a drug developed by AstraZeneca which is a peripherally selective cannabinoid agonist, acting as a potent agonist at the CB1 receptor and a partial agonist at CB2. It has poor blood–brain barrier penetration, and so while it is an effective analgesic in animal tests, it produces only peripheral effects at low doses, with much weaker symptoms of central effects compared to other cannabinoid drugs such as WIN 55,212-2. Many related benzimidazole-derived cannabinoid ligands are known.

CBS-0550 is a drug developed by Taisho Pharmaceutical, which acts as a potent and selective cannabinoid CB2 receptor agonist, with 1400x selectivity for CB2 over the related CB1 receptor. Unlike most cannabinoid agonists, CBS-0550 has good solubility in water, and in animal studies it was found to produce analgesic and anti-hyperalgesic effects. A number of related compounds have been developed with similar properties.
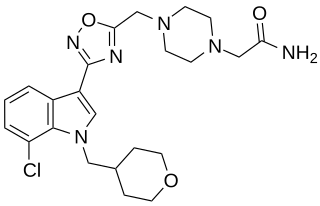
LBP-1 is a drug originally developed by Organon for the treatment of neuropathic pain, It acts as a potent and selective cannabinoid receptor agonist, with high potency at both the CB1 and CB2 receptors, but low penetration of the blood–brain barrier. This makes LBP-1 peripherally selective, and while it was effective in animal models of neuropathic pain and allodynia, it did not produce cannabinoid-appropriate responding suggestive of central effects, at any dose tested.

S-444,823 is a drug developed by Shionogi which is a cannabinoid agonist. It was developed as an antipruritic, and has moderate selectivity for the CB2 subtype, having a CB2 affinity of 18nM, and 32x selectivity over the CB1 receptor. In animal studies it showed analgesic effects and strongly reduced itching responses, but without producing side effects such as sedation and catalepsy that are seen with centrally acting CB1 agonists.

Cebranopadol is an opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.

Olorinab (APD371) is a drug being developed by Arena Pharmaceuticals for the treatment of gastrointestinal pain associated with Crohn's disease and irritable bowel syndrome. It acts as a potent and selective cannabinoid CB2 receptor agonist and is claimed to be orally active and peripherally selective. Initial Phase IIa exploratory clinical trials have been successful in patients with quiescent Crohn's disease. Arena initiated the Phase IIb Captivate trial in late July 2019 in patients with irritable bowel syndrome related pain, in constipation and diarrhea predominant sub-types. The Phase IIb trial is expected to enroll 240 participants between the ages of 18 and 70.Three doses of 10 mg, 25 mg, and 50 mg are being tested against Placebo in a 3:4 prescription ratio with a Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) masking layout.
The β3 adrenergic receptor agonist or β3-adrenoceptor agonist, also known as β3-AR agonist, are a class of medicine that bind selectively to β3-adrenergic receptors.

MCHB-1 is a benzimidazole derived drug which was researched as an analgesic but never developed for medical use. It acts as a potent agonist of the CB2 receptor, with an EC50 of 0.52nM at CB2, and ~30x selectivity over CB1 (Ki of 110nM at CB1 vs 3.7nM at CB2). It has been sold online as a designer drug, first being identified in Germany in December 2013.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
RQ-00202730 is a benzimidazole derived drug that acts as a potent and highly selective agonist for the CB2 cannabinoid receptor, with a Ki value of 19nM at CB2 and more than 4000x selectivity over CB1, though it also shows some activity as an antagonist of the unrelated 5-HT2B serotonin receptor. It has analgesic and antiinflammatory effects in animal studies, and was developed for the treatment of irritable bowel syndrome, but was ultimately discontinued from development following disappointing results in Phase II clinical trials.

















