
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates– a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands:

Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the CNR1 gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild daga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activate the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol.

The cannabinoid receptor 2(CB2), is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the CNR2 gene. It is closely related to the cannabinoid receptor 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids. The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG).

BAY 59-3074 is a drug which is a cannabinoid receptor partial agonist developed by Bayer AG. It has analgesic effects and is used in scientific research. It is orally active in animals, and has modest affinity for both CB1 and CB2 receptors, with Ki values of 48.3nM at CB1 and 45.5nM at CB2.
A cannabinoid receptor antagonist, also known simply as a cannabinoid antagonist or as an anticannabinoid, is a type of cannabinoidergic drug that binds to cannabinoid receptors (CBR) and prevents their activation by endocannabinoids. They include antagonists, inverse agonists, and antibodies of CBRs. The discovery of the endocannabinoid system led to the development of CB1 receptor antagonists. The first CBR inverse agonist, rimonabant, was described in 1994. Rimonabant blocks the CB1 receptor selectively and has been shown to decrease food intake and regulate body-weight gain. The prevalence of obesity worldwide is increasing dramatically and has a great impact on public health. The lack of efficient and well-tolerated drugs to cure obesity has led to an increased interest in research and development of CBR antagonists. Cannabidiol (CBD), a naturally occurring cannabinoid and a non-competitive CB1/CB2 receptor antagonist, as well as Δ9-tetrahydrocannabivarin (THCV), a naturally occurring cannabinoid, modulate the effects of THC via direct blockade of cannabinoid CB1 receptors, thus behaving like first-generation CB1 receptor inverse agonists, such as rimonabant. CBD is a very low-affinity CB1 ligand, that can nevertheless affect CB1 receptor activity in vivo in an indirect manner, while THCV is a high-affinity CB1 receptor ligand and potent antagonist in vitro and yet only occasionally produces effects in vivo resulting from CB1 receptor antagonism. THCV has also high affinity for CB2 receptors and signals as a partial agonist, differing from both CBD and rimonabant.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.
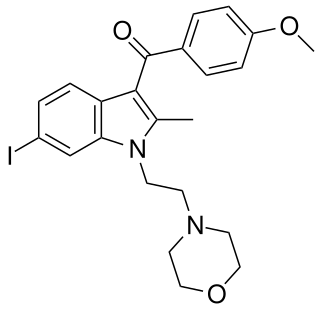
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a Ki of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (R) enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2.

AB-001 (1-pentyl-3-(1-adamantoyl)indole) is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011. It is unclear who AB-001 was originally developed by, but it is structurally related to compounds such as AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are known to be potent cannabinoid agonists with moderate to a high selectivity for CB2 over CB1. The first published synthesis and pharmacological evaluation of AB-001 revealed that it acts as a full agonist at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM). However, AB-001 was found to possess only weak cannabimimetic effects in rats at doses up to 30 mg/kg, making it less potent than the carboxamide analogue APICA, which possesses potent cannabimimetic activity at doses of 3 mg/kg.
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.

PF-514273 is a drug developed by Pfizer, which acts as an extremely selective antagonist for the CB1 receptor, with approximately 10,000x selectivity over the closely related CB2 receptor. This very high selectivity makes it useful for scientific research into these receptors, as many commonly used cannabinoid receptor antagonists also block the CB2 receptor to some extent.

AB-FUBINACA (AMB-FUBINACA) is a psychoactive drug that acts as a potent agonist for the cannabinoid receptors, with Ki values of 0.9 nM at CB1 and 23.2 nM at CB2 and EC50 values of 1.8 nM at CB1 and 3.2 nM at CB2. It was originally developed by Pfizer in 2009 as an analgesic medication but was never pursued for human use. In 2012, it was discovered as an ingredient in synthetic cannabinoid blends in Japan, along with a related compound AB-PINACA, which had not previously been reported.

ADBICA (also known as ADB-PICA) is a designer drug identified in synthetic cannabis blends in Japan in 2013. ADBICA had not previously been reported in the scientific literature prior to its sale as a component of synthetic cannabis blends. ADBICA features a carboxamide group at the 3-indole position, like SDB-001 and STS-135. The stereochemistry of the tert-butyl side-chain in the product is unresolved, though in a large series of indazole derivatives structurally similar to ADBICA that are disclosed in Pfizer patent WO 2009/106980, activity resides exclusively in the (S) enantiomers. ADBICA is a potent agonist of the CB1 receptor and CB2 receptor with an EC50 value of 0.69 nM and 1.8 nM respectively.

ADB-FUBINACA (ADMB-FUBINACA) is a designer drug identified in synthetic cannabis blends in Japan in 2013. In 2018, it was the third-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

5F-AB-PINACA is an indazole-based synthetic cannabinoid that is derived from a series of compounds originally developed by Pfizer in 2009 as an analgesic medication, and has been sold online as a designer drug.

S-777,469 is a drug developed by Shionogi which is a cannabinoid receptor agonist, with 128x selectivity for the CB2 subtype, having a CB2 affinity of 36nM, and a CB1 affinity over 4600nM.

CUMYL-FUBINACA (SGT-149) is an indazole-3-carboxamide based synthetic cannabinoid receptor agonist, with an EC50 of 1.8nM for human CB1 receptors and 23.7nM for human CB2 receptors, giving it around 13x selectivity for CB1. It has been sold online as a designer drug.
















