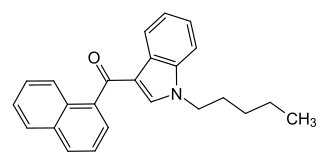
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends".

JWH-073, a synthetic cannabinoid, is an analgesic chemical from the naphthoylindole family that acts as a partial agonist at both the CB1 and CB2 cannabinoid receptors. It is somewhat selective for the CB1 subtype, with affinity at this subtype approximately 5x the affinity at CB2. The abbreviation JWH stands for John W. Huffman, one of the inventors of the compound.

JWH-147 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 11.0 nM at CB1 vs 7.1 nM at CB2. It was discovered and named after John W. Huffman.

JWH-307 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 7.7 nM at CB1 vs 3.3 nM at CB2. It was discovered by, and named after, John W. Huffman. JWH-307 was detected as an ingredient in synthetic cannabis smoking blends in 2012, initially in Germany.

JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a Ki of 11 nM at CB1 and 33 nM at CB2. Unlike many of the older JWH series compounds, this compound does not have a naphthalene ring, instead occupying this position with a 2'-methoxy-phenylacetyl group, making JWH-250 a representative member of a new class of cannabinoid ligands. Other 2'-substituted analogues such as the methyl, chloro and bromo compounds are also active and somewhat more potent.

Synthetic cannabinoids are a class of designer drug molecules that bind to the same receptors to which cannabinoids in cannabis plants attach. These novel psychoactive substances should not be confused with synthetic phytocannabinoids or synthetic endocannabinoids from which they are in many aspects distinct.

JWH-203 (1-pentyl-3-(2-chlorophenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist with approximately equal affinity at both the CB1 and CB2 receptors, having a Ki of 8.0 nM at CB1 and 7.0 nM at CB2. It was originally discovered by, and named after, John W. Huffman, but has subsequently been sold without his permission as an ingredient of synthetic cannabis smoking blends. Similar to the related 2'-methoxy compound JWH-250, the 2'-bromo compound JWH-249, and the 2'-methyl compound JWH-251, JWH-203 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds, and has the strongest in vitro binding affinity for the cannabinoid receptors of any compound in the phenylacetyl group.

JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substituted naphthoyl derivatives in the naphthoylindole series, having a higher binding affinity (i.e. lower Ki) at CB1 than both its 4-methyl and 4-n-propyl homologues JWH-122 (CB1 Ki 0.69 nM) and JWH-182 (CB1 Ki 0.65 nM) respectively, and than the 4-methoxy compound JWH-081 (CB1 Ki 1.2 nM). It was discovered by and named after John W. Huffman.
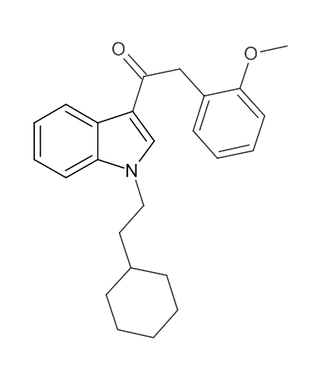
RCS-8 (also known as 1-(2-cyclohexylethyl)-3-(2-methoxyphenylacetyl)indole, SR-18, and BTM-8) is a synthetic cannabinoid that has been found as an ingredient of "herbal" synthetic cannabis blends. It can be described as an analogue of JWH-250 with the 1-pentyl group replaced by 1-(2-cyclohexylethyl), and can be expected to be less potent than JWH-250 (cf. JWH-007 and its cyclohexylethyl analogue). Despite not having been reported in the scientific or patent literature as yet, reputed recreational use of RCS-8 in the United States has led to it being specifically listed in a proposed 2011 amendment to the Controlled Substances Act, aiming to add a number of synthetic drugs into Schedule I. In addition, all CB1 receptor agonists of the 3-phenylacetylindole class such as RCS-8 are Schedule I Controlled Substances.
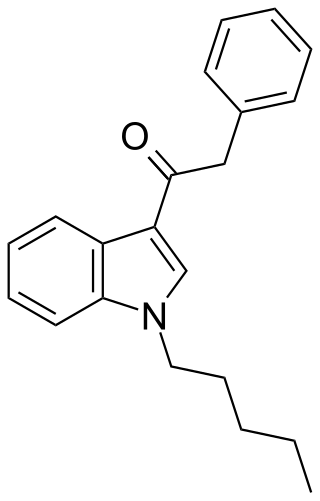
JWH-167 (1-pentyl-3-(phenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 1.75 times selectivity for CB1 with a Ki of 90 nM ± 17 and 159 nM ± 14 at CB2. Similar to the related 2'-methoxy compound JWH-250, and the 2'-chloro compound JWH-203, JWH-167 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.
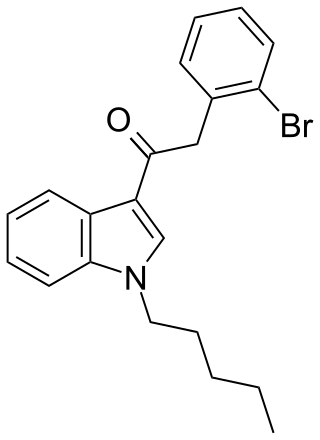
JWH-249 (1-pentyl-3-(2-bromophenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 2.4 times selectivity for CB1 with a Ki of 8.4 ± 1.8 nM and 20 ± 2 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-methyl compound JWH-251, JWH-249 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

AM-2201 is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University.
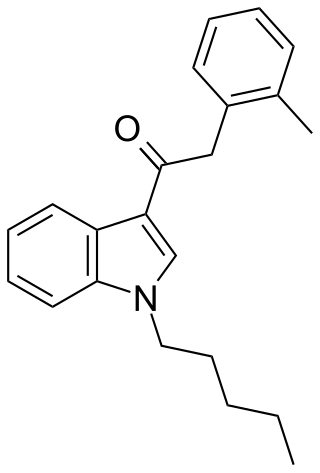
JWH-251 (1-pentyl-3-(2-methylphenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about five times selectivity for CB1 with a Ki of 29 nM and 146 nM at CB2. Similar to the related 2'-methoxy compound JWH-250, the 2'-chloro compound JWH-203, and the 2'-bromo compound JWH-249, JWH-251 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

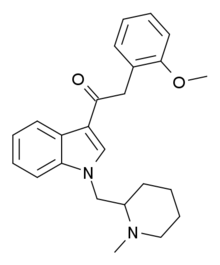
AM-1248 is a drug that acts as a moderately potent agonist for both the cannabinoid receptors CB1 and CB2, but with some dispute between sources over its exact potency and selectivity. Replacing the 3-(1-naphthoyl) group found in many indole derived cannabinoid ligands, with an adamantoyl group, generally confers significant CB2 selectivity, but reasonable CB1 affinity and selectivity is retained when an N-methylpiperidin-2-ylmethyl substitution is used at the indole 1-position. The related compound 1-pentyl-3-(1-adamantoyl)indole was identified as having been sold as a cannabinoid designer drug in Hungary in 2011, along with another synthetic cannabinoid AM-679.
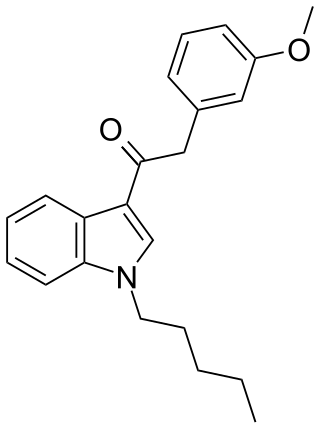
JWH-302 (1-pentyl-3-(3-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family, which acts as a cannabinoid agonist with moderate affinity at both the CB1 and CB2 receptors. It is a positional isomer of the more common drug JWH-250, though it is slightly less potent with a Ki of 17 nM at CB1, compared to 11 nM for JWH-250. Because of their identical molecular weight and similar fragmentation patterns, JWH-302 and JWH-250 can be very difficult to distinguish by GC-MS testing.
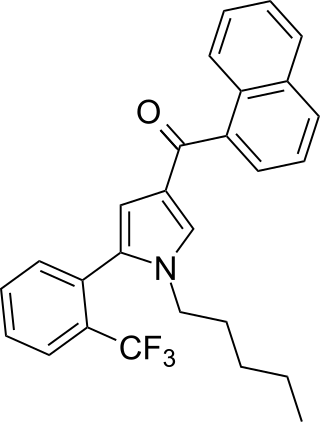
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).

JWH-371 ([5-(4-butylphenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 42 ± 1nM) and CB2 (Ki = 64 ± 2nM) receptors, binding ~1.5 times stronger to the CB1 receptor than the CB2 receptor. JWH-371 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-368 ([5-(3-Fluorophenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 16 ± 1nM) and CB2 (Ki = 9.1 ± 0.7nM) receptors, binding ~1.76 times stronger to the CB2 receptor than to the CB1 receptor. JWH-368 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-367 ([5-(3-methoxyphenyl)-1-pentylpyrrol-3-yl]-naphthalen-1-ylmethanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 53 ± 2nM) and CB2 (Ki = 23 ± 1nM) receptors, binding ~2.3 times stronger to the CB2 receptor than to the CB1 receptor. JWH-367 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-146 (1-heptyl-5-phenyl-1H-pyrrol-3-yl)-1-naphthalenyl-methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 21 ± 2nM) and CB2 (Ki = 62 ± 5nM) receptors, with a moderate (~2.9x) selectivity for the CB1 receptor over the CB2 receptor. JWH-146 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.