alpha/beta-Hydrolase domain containing 6 (ABHD6), also known as monoacylglycerol lipase ABHD6 or 2-arachidonoylglycerol hydrolase is an enzyme that in humans is encoded by the ABHD6 gene.
alpha/beta-Hydrolase domain containing 6 (ABHD6), also known as monoacylglycerol lipase ABHD6 or 2-arachidonoylglycerol hydrolase is an enzyme that in humans is encoded by the ABHD6 gene.
ABHD6 is a serine hydrolyzing enzyme that possesses typical α/β-hydrolase family domains. ABHD6 was first studied because of its over-expression in certain forms of tumours. [5]
ABHD6 has been linked to regulation of the endocannabinoid system as it controls the accumulation of 2-arachidonoylglycerol (2-AG) at the cannabinoid receptors. [6]
ABHD6 accounts for about 4% of 2-AG brain hydrolysis. [7] Together, monoacylglycerol lipase (MAGL), ABHD12, and ABHD6 control about 99% of 2-AG signalling in the brain, [7] [8] and each enzyme exhibits a distinct subcellular distribution, suggesting that they regulate distinct pools of 2-AG in the nervous system. [9]
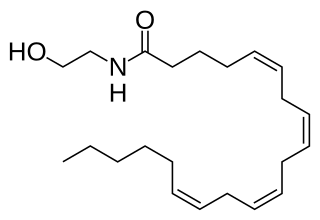
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), is a fatty acid neurotransmitter derived from the non-oxidative metabolism of eicosatetraenoic acid, an essential omega-6 fatty acid. The name is taken from the Sanskrit word ananda, which means "joy, bliss, delight", and amide. It is synthesized from N-arachidonoyl phosphatidylethanolamine by multiple pathways. It is degraded primarily by the fatty acid amide hydrolase (FAAH) enzyme, which converts anandamide into ethanolamine and arachidonic acid. As such, inhibitors of FAAH lead to elevated anandamide levels and are being pursued for therapeutic use.
The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors (CBRs), and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis.

Monoacylglycerol lipase, also known as MAG lipase, acylglycerol lipase, MAGL, MGL or MGLL is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.
Diacylglycerol lipase, also known as DAG lipase, DAGL or DGL, is a key enzyme in the biosynthesis of the endocannabinoid 2-arachidonoylglycerol. It catalyzes the hydrolysis of diacylglycerol, releasing a free fatty acid and monoacylglycerol.

Fatty acid amide hydrolase or FAAH is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide in 1993. In humans, it is encoded by the gene FAAH.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994-1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).
Serine hydrolases are one of the largest known enzyme classes comprising approximately ~200 enzymes or 1% of the genes in the human proteome. A defining characteristic of these enzymes is the presence of a nucleophilic serine in their active site, which is used for the hydrolysis of substrates. Catalysis proceeds by the formation of an acyl-enzyme intermediate through this serine, followed by water/hydroxide-induced saponification of the intermediate and regeneration of the enzyme. Unlike other non-catalytic serines, the nucleophilic serine of these hydrolases is typically activated by a proton relay involving a catalytic triad consisting of the serine, an acidic residue and a basic residue, although variations on this mechanism exist.

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor.

2-Arachidonyl glyceryl ether is a putative endocannabinoid discovered by Lumír Hanuš and colleagues at the Hebrew University of Jerusalem, Israel. It is an ether formed from the alcohol analog of arachidonic acid and glycerol. Its isolation from porcine brain and its structural elucidation and synthesis were described in 2001.

JZL184 is an irreversible inhibitor for monoacylglycerol lipase (MAGL), the primary enzyme responsible for degrading the endocannabinoid 2-arachidonoylglycerol (2-AG). It displays high selectivity for MAGL over other brain serine hydrolases, including the anandamide-degrading enzyme fatty acid amide hydrolase (FAAH), thereby making it a useful tool for studying the effects of endogenous 2-AG signaling, in vivo. Administration of JZL184 to mice was reported to cause dramatic elevation of brain 2-AG leading to several cannabinoid-related behavioral effects.

URB602 is a compound that has been found to inhibit hydrolysis of monoacyl glycerol compounds, such as 2-arachidonoylglycerol (2-AG) and 2-oleoylglycerol (2-OG). It was first described in 2003. A study performed in 2005 found that the compound had specificity for metabolizing 2-AG over anandamide in rat brain presumably by inhibiting the enzyme monoacylglycerol lipase (MAGL), which is the primary metabolic enzyme of 2-AG. However, subsequent studies have shown that URB602 lacks specificity for MAGL inhibition in vitro.

2-Oleoylglycerol (2OG) is a monoacylglycerol that is found in biologic tissues. Its synthesis is derived from diacylglycerol precursors. It is metabolized to oleic acid and glycerol primarily by the enzyme monoacylglycerol lipase (MAGL). In 2011, 2OG was found to be an endogenous ligand to GPR119. 2OG has been shown to increase glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) levels following administration to the small intestine.

Neutral cholesterol ester hydrolase 1 (NCEH) also known as arylacetamide deacetylase-like 1 (AADACL1) or KIAA1363 is an enzyme that in humans is encoded by the NCEH1 gene.

JZL195 is a potent inhibitor of both fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the primary enzymes responsible for degrading the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), respectively.

LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.
IDFP is an organophosphorus compound related to the nerve agent sarin. Like sarin, IDFP is an irreversible inhibitor for a number of different enzymes that normally serve to break down neurotransmitters, however the long alkyl chain of IDFP makes it dramatically weaker as an inhibitor of acetylcholinesterase (AChE), with an IC50 of only 6300 nM, while it is a potent inhibitor of two enzymes monoacylglycerol lipase (MAGL), the primary enzyme responsible for degrading the endocannabinoid 2-arachidonoylglycerol (2-AG), and fatty acid amide hydrolase (FAAH), the primary enzyme that degrades the other main endocannabinoid anandamide. The IC50 of IDFP is 0.8 nM at MAGL, and 3.0 nM at FAAH. Inhibition of these two enzymes causes markedly increased levels of both anandamide and 2-AG in the brain, resulting in increased cannabinoid signalling and typical cannabinoid behavioral effects in animal studies, while its lack of potency at AChE means that no cholinergic symptoms are produced.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.

Alpha/beta hydrolase domain containing 3 is a single pass type II membrane member of the serine hydrolase family of enzymes. The expression of murine ABHD3 is highest in the brain, liver, and kidney. ABHD3 hydrolytic activity is highly specific for medium chain and oxidatively truncated phospholipids. ABHD3-deficient mice are viable, fertile, and possess dramatically elevated medium chain phospholipids in tissues and in blood. Conversely, ectopic expression of ABHD3 prevents the accumulation of oxidized phospholipids in cells.
An endocannabinoid enhancer (eCBE) is a type of cannabinoidergic drug that enhances the activity of the endocannabinoid system by increasing extracellular concentrations of endocannabinoids. Examples of different types of eCBEs include fatty acid amide hydrolase (FAAH) inhibitors, monoacylglycerol lipase (MAGL) inhibitors, and endocannabinoid transporter (eCBT) inhibitors. An example of an actual eCBE is AM404, the active metabolite of the analgesic paracetamol and a dual FAAH inhibitor and eCBRI.

alpha/beta-Hydrolase domain containing 12 (ABHD12) is a serine hydrolase encoded by the ABHD12 gene that participates in the breakdown of the endocannabinoid neurotransmitter 2-arachidonylglycerol (2-AG) in the central nervous system. It is responsible for about 9% of brain 2-AG hydrolysis. Together, ABHD12 along with two other enzymes, monoacylglycerol lipase (MAGL) and ABHD6, control 99% of 2-AG hydrolysis in the brain. ABHD12 also serves as a lysophospholipase and metabolizes lysophosphatidylserine (LPS).
| This article on a gene on human chromosome 3 is a stub. You can help Wikipedia by expanding it. |