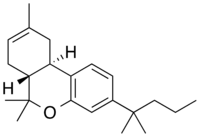
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure.

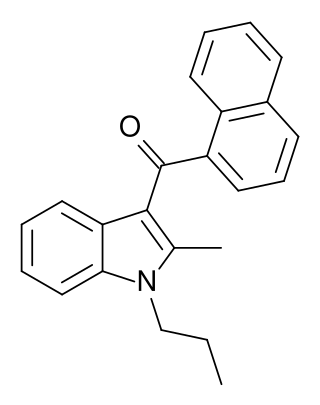
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at approximately 10x the affinity at CB2(12.4nM). It was discovered by and named after John W. Huffman.
JWH-015 is a chemical from the naphthoylindole family that acts as a subtype-selective cannabinoid agonist. Its affinity for CB2 receptors is 13.8 nM, while its affinity for CB1 is 383 nM, meaning that it binds almost 28 times more strongly to CB2 than to CB1. However, it still displays some CB1 activity, and in some model systems can be very potent and efficacious at activating CB1 receptors, and therefore it is not as selective as newer drugs such as JWH-133. It has been shown to possess immunomodulatory effects, and CB2 agonists may be useful in the treatment of pain and inflammation. It was discovered and named after John W. Huffman.

JWH-147 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 11.0 nM at CB1 vs 7.1 nM at CB2. It was discovered and named after the renowned professor of organic chemistry John W. Huffman.

JWH-307 is an analgesic drug used in scientific research, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is somewhat selective for the CB2 subtype, with a Ki of 7.7 nM at CB1 vs 3.3 nM at CB2. It was discovered by, and named after, John W. Huffman. JWH-307 was detected as an ingredient in synthetic cannabis smoking blends in 2012, initially in Germany.

JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.

JWH-359 is a dibenzopyran "classical" cannabinoid drug, which is a potent and selective CB2 receptor agonist, with a Ki of 13.0 nM and selectivity of around 220 times for CB2 over CB1 receptors. It is related to other dibenzopyran CB2 agonists such as JWH-133 and L-759,656 but with a chiral side chain which has made it useful for mapping the shape of the CB2 binding site. It was discovered by, and named after, John W. Huffman.

JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of N-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the N-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other N-alkyl substituents were found to be active by Huffman's team including the n-butyl, n-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, with an optimum binding of 9.00 nM at CB1 and 2.94 nM at CB2, and JWH-007 displayed optimum binding of 9.50 nM at CB1 and 2.94 nM at CB2.

JWH-019 is an analgesic chemical from the naphthoylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It is the N-hexyl homolog of the more common synthetic cannabinoid compound JWH-018. Unlike the butyl homolog JWH-073, which is several times weaker than JWH-018, the hexyl homolog is only slightly less potent, although extending the chain one carbon longer to the heptyl homolog JWH-020 results in dramatic loss of activity. These results show that the optimum side chain length for CB1 binding in the naphthoylindole series is the five-carbon pentyl chain, shorter than in the classical cannabinoids where a seven-carbon heptyl chain produces the most potent compounds. This difference is thought to reflect a slightly different binding conformation adopted by the naphthoylindole compounds as compared to the classical cannabinoids, and may be useful in characterizing the active site of the CB1 and CB2 receptors.
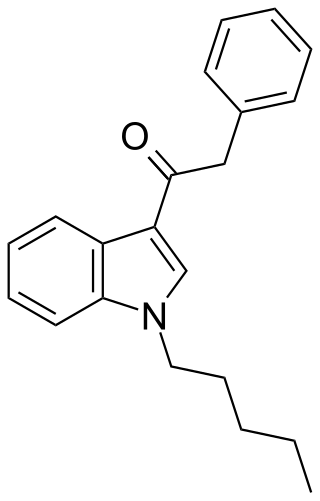
JWH-167 (1-pentyl-3-(phenylacetyl)indole) is a synthetic cannabinoid from the phenylacetylindole family, which acts as a cannabinoid agonist with about 1.75 times selectivity for CB1 with a Ki of 90 nM ± 17 and 159 nM ± 14 at CB2. Similar to the related 2'-methoxy compound JWH-250, and the 2'-chloro compound JWH-203, JWH-167 has a phenylacetyl group in place of the naphthoyl ring used in most aminoalkylindole cannabinoid compounds.

JWH-424 is a drug from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors, but with moderate selectivity for CB2, having a Ki of 5.44nM at CB2 vs 20.9 nM at CB1. The heavier 8-iodo analogue is even more CB2 selective, with its 2-methyl derivative having 40 times selectivity for CB2. However the 1-propyl homologues in this series showed much lower affinity at both receptors, reflecting a generally reduced affinity for the 8-substituted naphthoylindoles overall.

KM-233 is a synthetic cannabinoid drug which is a structural analog of Δ8-tetrahydrocannabinol (THC), the less active but more stable isomer of the active component of Cannabis. KM-233 differs from Δ8-THC by the pentyl side chain being replaced by a 1,1-dimethylbenzyl group. It has high binding affinity in vitro for both the CB1 and CB2 receptors, with a CB2 affinity of 0.91 nM and 13-fold selectivity over the CB1 receptor. In animal studies, it has been found to be a potential treatment for glioma, a form of brain tumor. Many related analogues are known where the 1,1-dimethylbenzyl group is substituted or replaced by other groups, with a fairly well established structure-activity relationship.
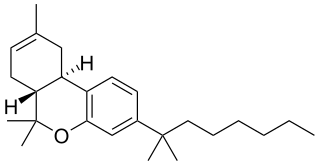
JWH-057, also known as deoxy-Δ8-THC-DMH, is a selective cannabinoid ligand, with a binding affinity of Ki = 2.9 ± 1.6 nM for the CB2 subtype, and Ki = 23 ± 7 nM for CB1.
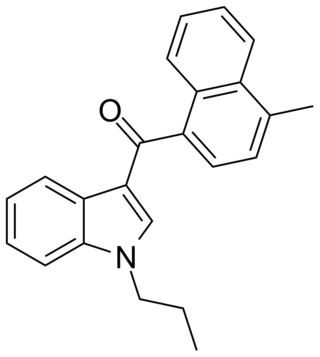
JWH-120 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-propyl analog of JWH-122. It is a potent and selective ligand for the CB2 receptor, but a weaker ligand for the CB1 receptor. It has a binding affinity of Ki = 6.1 ± 0.7 nM at the CB2 subtype and 173 times selectivity over the CB1 subtype.
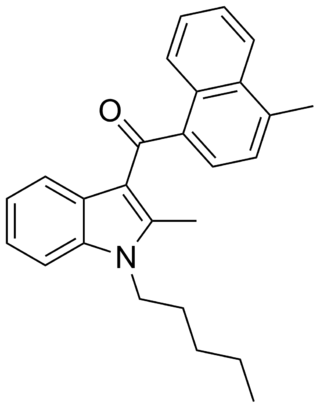
JWH-149 is a synthetic cannabimimetic that was discovered by John W. Huffman. It is the N-pentyl analog of JWH-148. It is a potent but only moderately selective ligand for the CB2 receptor, with a binding affinity of Ki = 0.73 ± 0.03 nM at this subtype, and more than six times selectivity over the CB1 subtype.
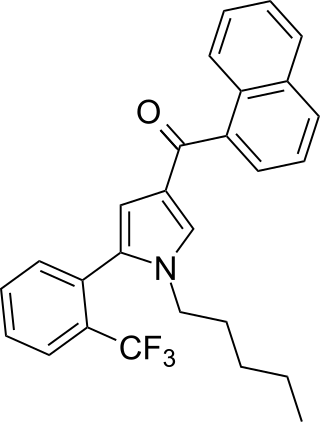
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).

JWH-366 (naphthalen-1-yl-(1-pentyl-5-pyridin-3-ylpyrrol-3-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 191 ± 12nM) and CB2 (Ki = 24 ± 1nM) receptors, with a strong (~8x) selectivity for the CB2 receptor over the CB1 receptor. JWH-366 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-365 ((5-(2-Ethylphenyl)-1-pentyl-1H-pyrrol-3-yl)(naphthalen-1-yl)methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 17 ± 1nM) and CB2 (Ki = 3.4 ± 0.2nM) receptors, with a strong (~5x) selectivity for the CB2 receptor over the CB1 receptor. JWH-365 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.

JWH-146 (1-heptyl-5-phenyl-1H-pyrrol-3-yl)-1-naphthalenyl-methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 21 ± 2nM) and CB2 (Ki = 62 ± 5nM) receptors, with a moderate (~2.9x) selectivity for the CB1 receptor over the CB2 receptor. JWH-146 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.