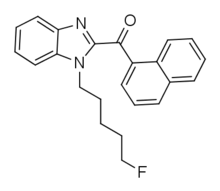
MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.
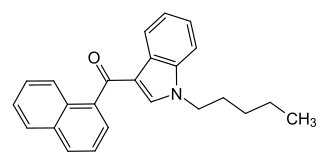
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use as synthetic cannabinoid products that, in some countries, are sold legally as "incense blends".

AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.

AM-2201 is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University.

AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a Ki of 0.28 nM at CB1 and 1.48 nM at CB2.

UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.

EAM-2201 is a drug that presumably acts as a potent agonist for the cannabinoid receptors. It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in July 2012 as an ingredient in synthetic cannabis smoking blends Like the closely related MAM-2201 which had been first reported around a year earlier, EAM-2201 thus appears to be another novel compound invented by designer drug suppliers specifically for recreational use. Structurally, EAM-2201 is a hybrid of two known cannabinoid compounds JWH-210 and AM-2201, both of which had previously been used as active ingredients in synthetic cannabis blends before being banned in many countries.

APICA is an indole based drug that acts as a potent agonist for the cannabinoid receptors.

PB-22 is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. PB-22 represents a structurally unique synthetic cannabinoid chemotype, since it contains an ester linker at the indole 3-position, rather than the precedented ketone of JWH-018 and its analogs, or the amide of APICA and its analogs.

5F-PB-22 is a designer drug which acts as a cannabinoid agonist. The structure of 5F-PB-22 appears to have been designed with an understanding of structure–activity relationships within the indole class of cannabinoids.

SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors. It was discovered during research into the related compound SDB-001 which had been sold illicitly as "2NE1". SDB-006 metabolism has been described in literature.

THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug.

MEPIRAPIM is an indole-based cannabinoid which differs from JWH-018 by having a 4-methylpiperazine group in place of the naphthyl group and has been used as an active ingredient in synthetic cannabis products. It was first identified in Japan in 2013, alongside FUBIMINA. MEPIRAPIM acts as a T-type calcium channel inhibitor and is only minimally active at the central CB1 receptor.
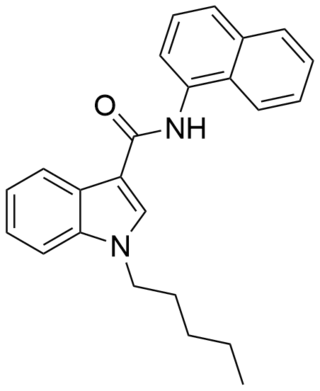
NNE1 (also known as NNEI, MN-24 and AM-6527) is an indole-based synthetic cannabinoid, representing a molecular hybrid of APICA and JWH-018 that is an agonist for the cannabinoid receptors, with Ki values of 60.09 nM at CB1 and 45.298 nM at CB2 and EC50 values of 9.481 nM at CB1 and 1.008 nM at CB2. It was invented by Abbott and has a CB1 receptor pEC50 of 8.9 with around 80x selectivity over the related CB2 receptor. It is suspected that metabolic hydrolysis of the amide group of NNE1 may release 1-naphthylamine, a known carcinogen, given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, and NNE1 was banned in New Zealand in 2012 as a temporary class drug to stop it being used as an ingredient in then-legal synthetic cannabis products. NNE1 was subsequently found to be responsible for the death of a man in Japan in 2014.

THJ-018 (SGT-17) is a synthetic cannabinoid that is the indazole analogue of JWH-018 and has been sold online as a designer drug.
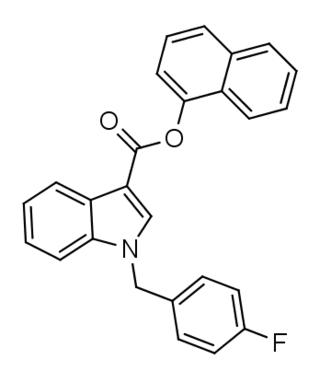
FDU-PB-22 is a derivative of JWH-018 that is presumed to be a potent agonist of the CB1 receptor, and has been sold online as a designer drug.
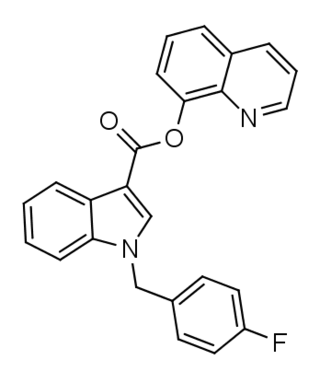
FUB-PB-22 (QUFUBIC) is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug.

BIM-018 is a synthetic cannabinoid that is the benzimidazole analog of JWH-018. It is presumed to be a potent agonist of the CB2 receptor and has been sold online as a designer drug.
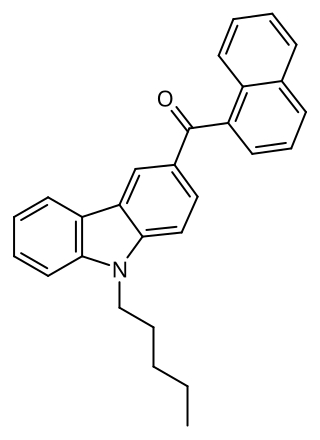
EG-018 is a carbazole-based synthetic cannabinoid that has been sold online as a designer drug. It acts as a partial agonist of the CB1 and CB2 receptor, with reasonably high binding affinity, but low efficacy in terms of inducing a signaling response.