
JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.
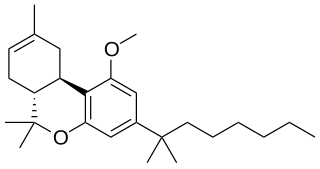
L-759,633 is an analgesic drug that is a cannabinoid agonist. It is a fairly selective agonist for the CB2 receptor, with selectivity of 163x for CB2 over CB1.

L-759,656 is an analgesic drug that is a cannabinoid agonist. It is a highly selective agonist for the CB2 receptor, with selectivity of 414x for CB2 over CB1, although it is still not as selective as newer agents such as HU-308.

NESS-0327 is a drug used in scientific research which acts as an extremely potent and selective antagonist of the cannabinoid receptor CB1. It is much more potent an antagonist, and more selective for the CB1 receptor over CB2, than the more commonly used ligand rimonabant, with a Ki at CB1 of 350fM (i.e. 0.00035nM) and a selectivity of over 60,000x for CB1 over CB2. Independently, two other groups have described only modest nanomolar CB1 affinity for this compound (125nM and 18.4nM). Also unlike rimonabant, NESS-0327 does not appear to act as an inverse agonist at higher doses, instead being a purely neutral antagonist which blocks the CB1 receptor but does not produce any physiological effect of its own.

JTE-907 is a drug used in scientific research that acts as a selective CB2 inverse agonist. It has antiinflammatory effects in animal studies, thought to be mediated by an interaction between the CB2 receptor and IgE.

JWH-359 is a dibenzopyran "classical" cannabinoid drug, which is a potent and selective CB2 receptor agonist, with a Ki of 13.0 nM and selectivity of around 220 times for CB2 over CB1 receptors. It is related to other dibenzopyran CB2 agonists such as JWH-133 and L-759,656 but with a chiral side chain which has made it useful for mapping the shape of the CB2 binding site. It was discovered by, and named after, John W. Huffman.

GW-405,833 (L-768,242) is a drug that acts as a potent and selective partial agonist for the cannabinoid receptor subtype CB2, with an EC50 of 0.65 nM and selectivity of around 1200x for CB2 over CB1 receptors. Animal studies have shown it to possess antiinflammatory and anti-hyperalgesic effects at low doses, followed by ataxia and analgesic effects when the dose is increased. Selective CB2 agonist drugs such as GW-405,833 are hoped to be particularly useful in the treatment of allodynia and neuropathic pain for which current treatment options are often inadequate.

AM-1241 (1-(methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole) is a chemical from the aminoalkylindole family that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 3.4 nM at CB2 and 80 times selectivity over the related CB1 receptor. It has analgesic effects in animal studies, particularly against "atypical" pain such as hyperalgesia and allodynia. This is thought to be mediated through CB2-mediated peripheral release of endogenous opioid peptides, as well as direct activation of the TRPA1 channel. It has also shown efficacy in the treatment of amyotrophic lateral sclerosis in animal models.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.

A-796,260 is a drug developed by Abbott Laboratories that acts as a potent and selective cannabinoid CB2 receptor agonist. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group, imparts significant selectivity for CB2, and A-796,260 was found to be a highly selective CB2 agonist with little affinity for CB1, having a CB2Ki of 4.6 nM vs 945 nM at CB1. It has potent analgesic and anti-inflammatory actions in animal models, being especially effective in models of neuropathic pain, but without producing cannabis-like behavioral effects.

AM-1221 is a drug that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 0.28 nM at CB2 and 52.3 nM at the CB1 receptor, giving it around 180 times selectivity for CB2. The 2-methyl and 6-nitro groups on the indole ring both tend to increase CB2 affinity while generally reducing affinity at CB1, explaining the high CB2 selectivity of AM-1221. However, despite this relatively high selectivity for CB2, its CB1 affinity is still too strong to make it useful as a truly selective CB2 agonist, so the related compound AM-1241 is generally preferred for research purposes.
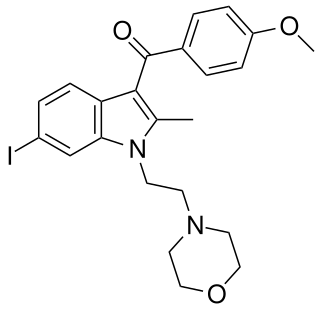
AM-630 (6-Iodopravadoline) is a drug that acts as a potent and selective inverse agonist for the cannabinoid receptor CB2, with a Ki of 32.1 nM at CB2 and 165x selectivity over CB1, at which it acted as a weak partial agonist. It is used in the study of CB2 mediated responses and has been used to investigate the possible role of CB2 receptors in the brain. AM-630 is significant as one of the first indole derived cannabinoid ligands substituted on the 6-position of the indole ring, a position that has subsequently been found to be important in determining affinity and efficacy at both the CB1 and CB2 receptors, and has led to the development of many related derivatives.

SR144528 is a drug that acts as a potent and highly selective CB2 receptor inverse agonist, with a Ki of 0.6 nM at CB2 and 400 nM at the related CB1 receptor. It is used in scientific research for investigating the function of the CB2 receptor, as well as for studying the effects of CB1 receptors in isolation, as few CB1 agonists that do not also show significant activity as CB2 agonists are available. It has also been found to be an inhibitor of sterol O-acyltransferase, an effect that appears to be independent from its action on CB2 receptors.

JWH-424 is a drug from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors, but with moderate selectivity for CB2, having a Ki of 5.44nM at CB2 vs 20.9 nM at CB1. The heavier 8-iodo analogue is even more CB2 selective, with its 2-methyl derivative having 40 times selectivity for CB2. However the 1-propyl homologues in this series showed much lower affinity at both receptors, reflecting a generally reduced affinity for the 8-substituted naphthoylindoles overall.

AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a Ki of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action. Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2. High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812.
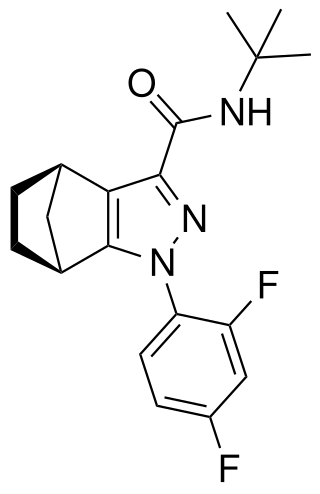
Tedalinab (GRC-10693) is a drug developed by Glenmark Pharmaceuticals for the treatment of osteoarthritis and neuropathic pain, which acts as a potent and selective cannabinoid CB2 receptor agonist. It has a very high selectivity of 4700x for CB2 over the related CB1 receptor, has good oral bioavailability and has shown promising safety results and effective analgesic and antiinflammatory actions in early clinical trials. Many related compounds are known, most of which also show high CB2 selectivity.

AM-1714 (part of the AM cannabinoid series) is a drug that acts as a reasonably selective agonist of the peripheral cannabinoid receptor CB2, with sub-nanomolar affinity and 490x selectivity over the related CB1 receptor. In animal studies it has both analgesic and anti-allodynia effects. The 9-methoxy derivative AM-1710 has similar CB2 affinity but only 54x selectivity over CB1.

Olorinab (APD371) is a drug being developed by Arena Pharmaceuticals for the treatment of gastrointestinal pain associated with Crohn's disease and irritable bowel syndrome. It acts as a potent and selective cannabinoid CB2 receptor agonist and is claimed to be orally active and peripherally selective. Initial Phase IIa exploratory clinical trials have been successful in patients with quiescent Crohn's disease. Arena initiated the Phase IIb Captivate trial in late July 2019 in patients with irritable bowel syndrome related pain, in constipation and diarrhea predominant sub-types. The Phase IIb trial is expected to enroll 240 participants between the ages of 18 and 70.Three doses of 10 mg, 25 mg, and 50 mg are being tested against Placebo in a 3:4 prescription ratio with a Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) masking layout.

Cannabinor (PRS-211,375) is a drug which acts as a potent and selective cannabinoid CB2 receptor agonist. It is classed as a "nonclassical" cannabinoid with a chemical structure similar to that of cannabidiol. It has a CB2 affinity of 17.4 nM vs 5,585 nM at CB1, giving it over 300× selectivity for CB2. It showed analgesic effects in animal studies especially in models of neuropathic pain, but failed in Phase IIb human clinical trials due to lack of efficacy.
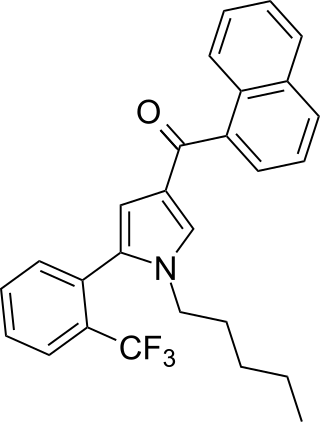
JWH-372 (naphthalen-1-yl-[1-pentyl-5-[2-(trifluoromethyl)phenyl]pyrrol-3-yl]methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as a potent and selective agonist of the CB2 receptor. JWH-372 binds approximately 9 times stronger to the CB2 receptor (Ki = 8.2 ± 0.2nM) than the CB1 receptor (Ki = 77 ± 2nM). The selectivity of JWH-372 for the CB2 receptor is likely due to the electron-withdrawing character of the trifluoromethyl group rather than steric effects, as the o-methyl compound JWH-370 was only mildly selective for the CB2 receptor (CB1 Ki = 5.6 ± 0.4nM, CB2 Ki = 4.0 ± 0.5nM).




















