5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membranes of synaptic vesicles of presynaptic neurons. It transports monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.
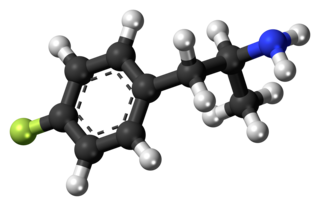
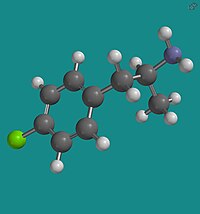
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.
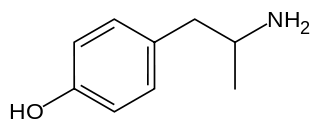
4-Hydroxyamphetamine (4HA), also known as hydroxyamfetamine, hydroxyamphetamine, oxamphetamine, norpholedrine, para-hydroxyamphetamine, and α-methyltyramine, is a drug that stimulates the sympathetic nervous system.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene. TAAR1 is an intracellular amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells, astrocytes, and in the intracellular milieu within the presynaptic plasma membrane of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al. TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms.

5,7-Dihydroxytryptamine (5,7-DHT) is a purported neurotoxin used in scientific research to decrease concentrations of serotonin in the brain. The mechanism behind this effect is not well understood, but it is speculated to selectively destroy serotonergic neurons, in a manner similar to the dopaminergic neurotoxicity of 6-hydroxydopamine (6-OHDA). What is known is that this compound is in fact not selective in depleting serotonin content, but also depletes norepinephrine. To selectively deplete serotonin stores, it is commonly administered in conjunction with desmethylimipramine (desipramine), which inhibits the norepinephrine transporter.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.

5-Iodo-2-aminoindane (5-IAI) is a drug which acts as a releasing agent of serotonin, norepinephrine, and dopamine. It was developed in the 1990s by a team led by David E. Nichols at Purdue University. 5-IAI fully substitutes for MDMA in rodents and is a putative entactogen in humans. Unlike related aminoindane derivatives like MDAI and MMAI, 5-IAI causes some serotonergic neurotoxicity in rats, but is substantially less toxic than its corresponding amphetamine homologue pIA, with the damage observed barely reaching statistical significance.

2-Aminotetralin (2-AT), also known as 1,2,3,4-tetrahydronaphthalen-2-amine (THN), is a stimulant drug with a chemical structure consisting of a tetralin group combined with an amine.

para-Bromoamphetamine (PBA), also known as 4-bromoamphetamine (4-BA), is an amphetamine derivative which acts as a serotonin-norepinephrine-dopamine releasing agent (SNDRA) and produces stimulant effects.

para-Iodoamphetamine (PIA), also known as 4-iodoamphetamine (4-IA), is a research chemical of the phenethylamine and amphetamine chemical classes.

Amiflamine (FLA-336) is a reversible inhibitor of monoamine oxidase A (MAO-A), thereby being a RIMA, and, to a lesser extent, semicarbazide-sensitive amine oxidase (SSAO), as well as a serotonin releasing agent (SRA). It is a derivative of the phenethylamine and amphetamine chemical classes. The (+)-enantiomer is the active stereoisomer.
EXP-561 is an investigational drug that acts as an inhibitor of the reuptake of serotonin, dopamine, and norepinephrine. It was developed in the 1960s by Du Pont and was suggested as a potential antidepressant but failed in trials and was never marketed.

4-Chlorophenylisobutylamine, also known as 4-chloro-α-ethylphenethylamine, is an entactogen and stimulant drug of the phenethylamine class. It is an analogue of para-chloroamphetamine (PCA) where the alpha position methyl has been replaced with an ethyl group.

3,4-Dichloroamphetamine (DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related para-chloroamphetamine, though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine N-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.
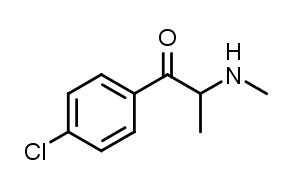
4-Chloromethcathinone is a stimulant drug of the cathinone class that has been sold online as a designer drug.