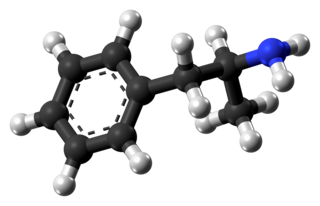
Amphetamine is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers, levoamphetamine and dextroamphetamine, in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.

Stimulants is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body, drugs that are pleasurable and invigorating, or drugs that have sympathomimetic effects. Stimulants are widely used throughout the world as prescription medicines as well as without a prescription as performance-enhancing or recreational drugs. Among narcotics, stimulants produce a noticeable crash or comedown at the end of their effects. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine, methylphenidate (Ritalin), and amphetamine. It was estimated in 2015 that the percentage of the world population that had used cocaine during a year was 0.4%. For the category "amphetamines and prescription stimulants" the value was 0.7%, and for Ecstasy 0.4%.

Methcathinone is a monoamine alkaloid and psychoactive stimulant, a substituted cathinone. It is used as a recreational drug due to its potent stimulant and euphoric effects and is considered to be addictive, with both physical and psychological withdrawal occurring if its use is discontinued after prolonged or high-dosage administration. It is usually snorted, but can be smoked, injected, or taken orally.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Phenylpropanolamine (PPA) is a sympathomimetic agent which is used as a decongestant and appetite suppressant. It was commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to control urinary incontinence in dogs.

2,5-Dimethoxy-4-ethylamphetamine is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL.
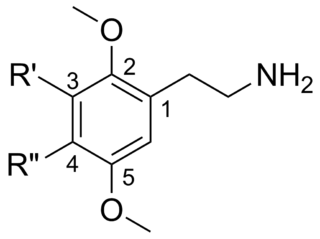
2C (2C-x) is a general name for the family of psychedelic phenethylamines containing methoxy groups on the 2 and 5 positions of a benzene ring. Most of these compounds also carry lipophilic substituents at the 4 position, usually resulting in more potent and more metabolically stable and longer acting compounds. Most of the currently known 2C compounds were first synthesized by Alexander Shulgin in the 1970s and 1980s and published in his book PiHKAL. Shulgin also coined the term 2C, being an acronym for the 2 carbon atoms between the benzene ring and the amino group.

Aleph is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine class of compounds, which can be used as an entheogen. It was first synthesized by Alexander Shulgin. In his book PiHKAL, Shulgin lists the dosage range as 5–10 mg. According to Shulgin, the effects of aleph typically last for 6 to 8 hours.

Substituted methylenedioxy- phenethylamines (MDxx) are a large chemical class of derivatives of the phenethylamines, which includes many psychoactive drugs that act as entactogens, psychedelics, and/or stimulants, as well as entheogens. These agents are used as research chemicals, designer drugs and as recreational substances.

2,5-Dimethoxy-4-propylamphetamine (DOPR) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL. Shulgin described DOPR is a "heavy duty psychedelic", complete with alterations of the thought process and visual distortion. Very little data exists about the pharmacological properties, metabolism, and toxicity of DOPR.

Etilamfetamine is a stimulant drug of the phenethylamine and amphetamine chemical classes. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced. It most likely acts primarily as a dopamine releasing agent. Its activity as a norepinephrine or serotonin releasing agent is not known.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene. TAAR1 is an intracellular amine-activated Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells, astrocytes, and in the intracellular milieu within the presynaptic plasma membrane of monoamine neurons in the central nervous system (CNS). TAAR1 was discovered in 2001 by two independent groups of investigators, Borowski et al. and Bunzow et al. TAAR1 is one of six functional human trace amine-associated receptors, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms.
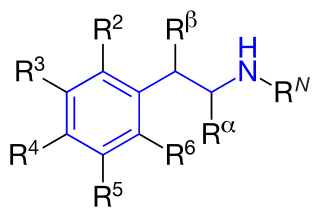
Substituted phenethylamines are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative compounds of phenethylamine which can be formed by replacing, or substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents.
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, bupropion, methoxyphenamine, selegiline, amfepramone, pyrovalerone, MDMA (ecstasy), and DOM (STP).
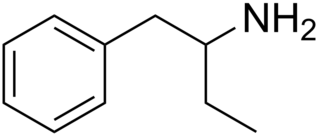
Phenylisobutylamine, also known as α-ethylphenethylamine, Butanphenamine, B or AEPEA, is a stimulant drug of the phenethylamine class. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group. Compared to amphetamine, phenylisobutylamine has strongly reduced dopaminergic effects, and instead acts as a selective norepinephrine releasing agent. The dextroisomer of phenylisobutylamine partially substitutes for dextroamphetamine in rats.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects though they retain amphetamine-like stimulant effects.

3-Fluoromethcathinone is a chemical compound of the phenethylamine, amphetamine, and cathinone classes that has been sold online as a designer drug. It is a structural isomer of flephedrone (4-fluoromethcathinone).
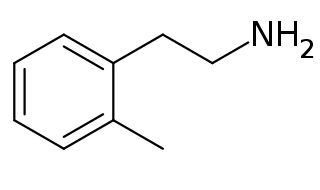
2-Methylphenethylamine (2MPEA) is an organic compound with the chemical formula of C9H13N. 2MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine).

3-Methylphenethylamine (3MPEA) is an organic compound with the chemical formula of C9H13N. 3MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine).

4-Methylphenethylamine (4MPEA), also known as para-methylphenethylamine, is an organic compound with the chemical formula of C9H13N. 4MPEA is a human trace amine associated receptor 1 (TAAR1) agonist, a property which it shares with its monomethylated phenethylamine isomers, such as amphetamine (α-methylphenethylamine), β-methylphenethylamine, and N-methylphenethylamine (a trace amine). 4MPEA also appears to inhibit the human cytochrome P450 enzymes CYP1A2 and CYP2A6, based upon the published literature.




















