Aniracetam, also known as N-anisoyl-2-pyrrolidinone, is a racetam which is sold in Europe as a prescription drug. It is not approved by the Food and Drug Administration for use in the United States as a prescription medication or dietary supplement. Despite the FDA's lack of approval, the drug is readily available over-the-counter in the US as a dietary supplement.
Ampakines, also stylized as AMPAkines, are a subgroup of AMPA receptor positive allosteric modulators with a benzamide or closely related chemical structure. They are also known as "CX compounds". Ampakines take their name from the AMPA receptor (AMPAR), a type of ionotropic glutamate receptor with which the ampakines interact and act as positive allosteric modulators (PAMs) of. Although all ampakines are AMPAR PAMs, not all AMPAR PAMs are ampakines.

CX717 is an ampakine compound created by Christopher Marrs and Gary Rogers in 1996 at Cortex Pharmaceuticals. It affects the neurotransmitter glutamate, with trials showing the drug improves cognitive functioning and memory.
Racetams are a class of drugs that share a pyrrolidone nucleus. Some, such as piracetam, aniracetam, oxiracetam, pramiracetam and phenylpiracetam are considered nootropics. Others such as levetiracetam, brivaracetam, and seletracetam are anticonvulsants.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

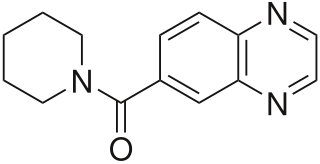
CX-516 is an ampakine and nootropic that acts as an AMPA receptor positive allosteric modulator and had been undergoing development by a collaboration between Cortex, Shire, and Servier. It was studied as a potential treatment for Alzheimer's disease under the brand name Ampalex, and was also being examined as a treatment for ADHD.

Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.
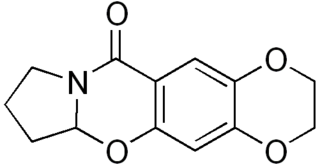
CX-614 is an ampakine drug developed by Cortex Pharmaceuticals. It has been investigated for its effect on AMPA receptors.
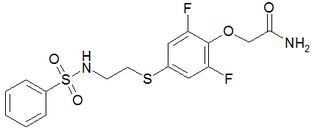
PEPA is a sulfonamide AMPA receptor positive allosteric modulator, which is up to 100 times more potent than aniracetam in vitro. It produces memory-enhancing effects in rats when administered intravenously.

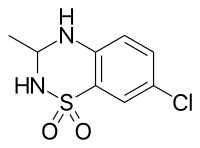
Cyclothiazide, sometimes abbreviated CTZ, is a benzothiadiazide (thiazide) diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly and was subsequently also marketed in Europe and Japan. Related drugs include diazoxide, hydrochlorothiazide, and chlorothiazide.

LY-404187 is an AMPA receptor positive allosteric modulator which was developed by Eli Lilly and Company. It is a member of the biarylpropylsulfonamide class of AMPA receptor potentiators.

5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used in vivo and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors in vitro. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato.
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimulus. Some of them, like benzodiazepines, are drugs. The site that an allosteric modulator binds to is not the same one to which an endogenous agonist of the receptor would bind. Modulators and agonists can both be called receptor ligands.

Sunifiram is an experimental drug which has antiamnesic effects in animal studies and with significantly higher potency than piracetam. Sunifiram is a molecular simplification of unifiram (DM-232). Another analogue is sapunifiram (MN-19). As of 2016, sunifiram had not been subjected to toxicology testing, nor to any human clinical trials, and is not approved for use anywhere in the world.

PNU-120596 is a drug that acts as a potent and selective positive allosteric modulator for the α7 subtype of neural nicotinic acetylcholine receptors. It is used in scientific research into cholinergic regulation of dopamine and glutamate release in the brain.
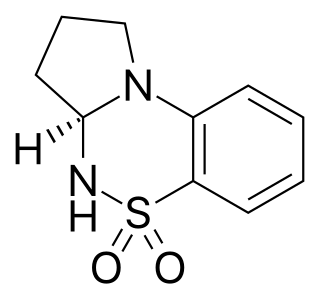
S-18986 is a positive allosteric modulator of the AMPA receptor related to cyclothiazide. It has nootropic and neuroprotective effects in animal studies, and induces both production of BDNF and AMPA-mediated release of noradrenaline and acetylcholine in the hippocampus and frontal cortex of the brain.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

BIIB-104, also known as PF-04958242, is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by Pfizer for the treatment of cognitive symptoms in schizophrenia. It was also under development for the treatment of age-related sensorineural hearing loss, but development for this indication was terminated due to insufficient effectiveness. As of July 2018, BIIB-104 is in phase II clinical trials for cognitive symptoms in schizophrenia.

Tulrampator is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by RespireRx Pharmaceuticals and Servier for the treatment of major depressive disorder, Alzheimer's disease, dementia, and mild cognitive impairment. Tulrampator was in phase II clinical trial for depression, but failed to show superiority over placebo. There are also phase II clinical trials for Alzheimer's disease and phase I trials for dementia and mild cognitive impairment.

AMPA receptor positive allosteric modulators are positive allosteric modulators (PAMs) of the AMPA receptor (AMPR), a type of ionotropic glutamate receptor which mediates most fast synaptic neurotransmission in the central nervous system.