A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.

Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an empathogen and stimulant psychoactive drug. It is a member of the amphetamine, cathinone and methylenedioxyphenethylamine classes.

Methylenedioxypyrovalerone (MDPV) is a stimulant of the cathinone class that acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim. Its activity at the dopamine transporter is six times stronger than at the norepinephrine transporter and it is virtually inactive at the serotonin transporter. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. In the US, products containing MDPV and labeled as bath salts were sold as recreational drugs in gas stations, similar to the marketing for Spice and K2 as incense, until it was banned in 2011.

Pyrovalerone is a psychoactive drug with stimulant effects via acting as a norepinephrine-dopamine reuptake inhibitor (NDRI), and is used for the clinical treatment of chronic fatigue or lethargy and as an anorectic or appetite suppressant for weight loss purposes. It was developed in the late 1960s and has since been used in France and several other European countries, and although pyrovalerone is still occasionally prescribed, it is used infrequently due to problems with abuse and dependence. It is closely related on a structural level to a number of other stimulants, such as α-PVP, MDPV and prolintane.

Methedrone is a recreational drug of the cathinone chemical class. Chemically, methedrone is closely related to para-methoxymethamphetamine (PMMA), methylone and mephedrone. Methedrone received media attention in 2009 after the death of two young Swedish men. In both cases toxicology analysis showed methedrone was the only drug present in both men during the time of their overdose and subsequent deaths.

4'-Methyl-α-pyrrolidinopropiophenone is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s, along with a number of other pyrrolidinophenone derivatives. Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends such as "NRG-3".

3',4'-Methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP) is a stimulant of the cathinone class developed in the 1960s, which has been reported as a novel designer drug. MDPBP is sometimes sold under the name "NRG-1" as a mixture with other cathinone derivatives, including flephedrone, pentylone, MαPPP and its higher homologue MDPV. As with other cathinones, MDPBP has been shown to have reinforcing effects in rats.

α-Pyrrolidinopentiophenone is a synthetic stimulant of the cathinone class developed in the 1960s that has been sold as a designer drug. Colloquially, it is sometimes called flakka. α-PVP is chemically related to pyrovalerone and is the ketone analog of prolintane.

Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

Pentylone is a stimulant developed in the 1960s. It is a substituted cathinone. It has been identified in some samples of powders sold as "NRG-1", along with varying blends of other cathinone derivatives including flephedrone, MDPBP, MDPV and 4-MePPP. It was also found in combination with 4-MePPP being sold as "NRG-3". Reports indicate side effects include feelings of paranoia, agitation and inability to sleep, with effects lasting for several days at high doses.
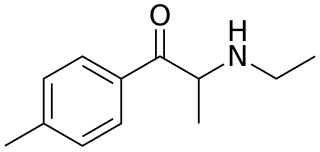
4-Methylethcathinone or 4-MEC is a chemical that bears a chemical resemblance to mephedrone. Due to its similarity to mephedrone, it is thought to be a stimulant and entactogen drug of the phenethylamine, amphetamine, and cathinone chemical classes. It has been marketed alone or in mixtures with other substituted cathinones under the name "NRG-2", although other blends such as "NRG-1" may have been more ambiguous with their ingredients.

3,4-Dimethylmethcathinone (3,4-DMMC) is a stimulant drug first reported in 2010 as a designer drug analogue of mephedrone, apparently produced in response to the banning of mephedrone, following its widespread abuse in many countries in Europe and around the world. 3,4-DMMC has been seized as a designer drug in Australia. In vitro, 3,4-DMMC was shown to be a monoamine transporter substrate that potently inhibits norepinephrine and serotonin reuptake, and to a lesser extent dopamine reuptake.

Pentedrone is a stimulant of the cathinone class that has been sold as a designer drug and has been found since 2010 as an ingredient in a number of "bath salt" mixes sold as legal highs.
Bath salts are a group of recreational designer drugs. The name derives from instances in which the drugs were disguised as bath salts. The white powder, granules, or crystals often resemble Epsom salts, but differ chemically. The drugs' packaging often states "not for human consumption" in an attempt to circumvent drug prohibition laws. Additionally, they may be mislabeled as plant food, powdered cleaner, and other such products.

3-Methylmethcathinone, also known as 3-MMC and metaphedrone, is a designer drug from the substituted cathinone family. 3-MMC is closely related in structure to the more commonly known illicit drug mephedrone (4-MMC), and is also illegal in most countries that have banned mephedrone due to 3-MMC being a structural isomer of 4-MMC. However, 3-MMC has still appeared on the recreational drug market as an alternative to mephedrone, and was first identified being sold in Sweden in 2012. Unlike some synthetic cathinones, 3-MMC has been evaluated in at least one large mammal study. 3-MMC is a monoamine transporter substrate that potently inhibits norepinephrine uptake and displays more pronounced dopaminergic vs. serotonergic activity.

3',4'-Dimethoxy-α-pyrrolidinopentiophenone is a synthetic stimulant drug of the cathinone class that has been sold online as a designer drug. It is a relatively weak inhibitor of serotonin reuptake and has little affinity in vitro for dopamine or noradrenaline transporters.

Mexedrone is a stimulant and an entactogen drug of the cathinone class that has been sold online as a designer drug. It is the alpha-methoxy derivative of Mephedrone.

α-PHiP is a stimulant drug of the cathinone class that has been sold online as a designer drug. It is a positional isomer of pyrovalerone, with the methyl group shifted from the 4-position of the aromatic ring to the 4-position of the acyl chain. In a classic 2006 study of pyrrolidinyl cathinone derivatives by Meltzer et al. at Organix, the alpha-isobutyl derivative of pyrovalerone, O-2494, was found to have the highest potency in vitro as an inhibitor of the dopamine transporter of the alpha substituted derivatives tested; however, it was not until ten years later in July 2016 that α-PHiP was first identified as a designer drug, when it was reported to the EMCDDA by a forensic laboratory in Slovenia.

BMAPN (βk-methamnetamine) is a substituted cathinone derivative with stimulant effects. It inhibits dopamine reuptake and has rewarding and reinforcing properties in animal studies. It is banned under drug analogue legislation in a number of jurisdictions. The drug was at one point marketed under the name NRG-3, although only a minority of samples of substances sold under this name have been found to actually contain BMAPN, with most such samples containing mixtures of other cathinone derivatives.

O-2390 is a recreational designer drug from the substituted cathinone family, which acts as a potent inhibitor of dopamine and noradrenaline reuptake in vitro, with weaker but still significant inhibition of serotonin reuptake.



















