
Sertraline, sold under the brand name Zoloft among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. The effectiveness of sertraline for depression is similar to that of other antidepressants, and the differences are mostly confined to side effects. Sertraline is better tolerated than the older tricyclic antidepressants. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder (GAD), and obsessive–compulsive disorder (OCD). Although approved for post-traumatic stress disorder (PTSD), sertraline leads to only modest improvement in this condition. Sertraline also alleviates the symptoms of premenstrual dysphoric disorder (PMDD) and can be used in sub-therapeutic doses or intermittently for its treatment.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are nonsuperposable onto their own mirror image. Enantiomers of each other are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. It is solely a relationship of chirality and the permanent three-dimensional relationships among molecules or other chemical structures: no amount of re-orientiation of a molecule as a whole or conformational change converts one chemical into its enantiomer. Chemical structures with chirality rotate plane-polarized light. A mixture of equal amounts of each enantiomer, a racemic mixture or a racemate, does not rotate light.
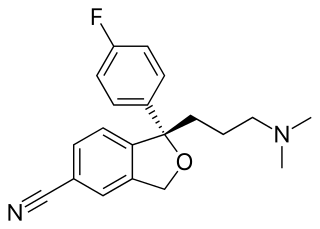
Escitalopram, sold under the brand names Lexapro and Cipralex, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Escitalopram is mainly used to treat major depressive disorder and generalized anxiety disorder. It is taken by mouth, available commercially as an oxalate salt exclusively.

Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia. The antidepressant effects may take one to four weeks to occur. It is typically taken orally. In some European countries, it is sometimes given intravenously to initiate treatment, before switching to the oral route of administration for continuation of treatment. It has also been used intravenously in other parts of the world in some other circumstances.
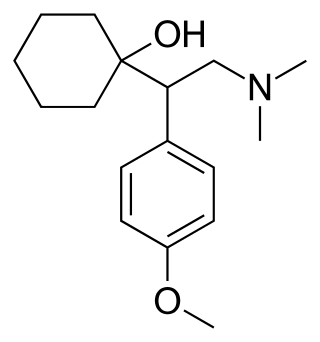
Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin–norepinephrine reuptake inhibitor (SNRI) class. It is used to treat major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder. Studies have shown that venlafaxine improves post-traumatic stress disorder (PTSD). It may also be used for chronic pain. It is taken orally. It is also available as the salt venlafaxine besylate in an extended-release formulation.
Forest Laboratories was a company in the pharmaceutical industry incorporated in Delaware, with its principal office in New York City. It was known for licensing European pharmaceuticals for sale in the United States. On July 1, 2014, the company was acquired by Actavis.

Ethyl loflazepate is a drug which is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. In animal studies it was found to have low toxicity, although in rats evidence of pulmonary phospholipidosis occurred with pulmonary foam cells developing with long-term use of very high doses. Its elimination half-life is 51–103 hours. Its mechanism of action is similar to other benzodiazepines. Ethyl loflazepate also produces an active metabolite which is stronger than the parent compound. Ethyl loflazepate was designed to be a prodrug for descarboxyloflazepate, its active metabolite. It is the active metabolite which is responsible for most of the pharmacological effects rather than ethyl loflazepate. The main metabolites of ethyl loflazepate are descarbethoxyloflazepate, loflazepate and 3-hydroxydescarbethoxyloflazepate. Accumulation of the active metabolites of ethyl loflazepate are not affected by those with kidney failure or impairment. The symptoms of an overdose of ethyl loflazepate include sleepiness, agitation and ataxia. Hypotonia may also occur in severe cases. These symptoms occur much more frequently and severely in children. Death from therapeutic maintenance doses of ethyl loflazepate taken for 2 – 3 weeks has been reported in 3 elderly patients. The cause of death was asphyxia due to benzodiazepine toxicity. High doses of the antidepressant fluvoxamine may potentiate the adverse effects of ethyl loflazepate.
Allosteric serotonin reuptake inhibitor is a type of selective serotonin reuptake inhibitor (SSRI).
A spontaneous orgasm, or spontaneous ejaculation when it occurs in males, is an orgasm which occurs spontaneously and involuntarily without sexual stimulation. Nocturnal emissions may be considered a normal/physiological form of spontaneous orgasm. Pathological spontaneous orgasms can be experienced as pleasurable, non-pleasurable, or unpleasant, and can be distressing. Causes of pathological spontaneous orgasms include spinal cord lesions, psychological causes, rabies, and medications. Some cases may have no identifiable cause. Spontaneous orgasms may have no trigger or may be triggered by various non-sexual circumstances. They may occur in both males and females. Treatment of spontaneous orgasms include psychotherapy, selective serotonin reuptake inhibitors (SSRIs), the alpha-1 blocker silodosin, and anxiolytics.

A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors.
The number of new psychiatric drugs, and especially antidepressants on the market in Japan, is significantly less than Western countries.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Desmethylcitalopram is an active metabolite of the antidepressant drugs citalopram (racemic) and escitalopram. Like citalopram and escitalopram, desmethylcitalopram functions as a selective serotonin reuptake inhibitor (SSRI), and is responsible for some of its parents' therapeutic benefits.

Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
Pharmacometabolomics, also known as pharmacometabonomics, is a field which stems from metabolomics, the quantification and analysis of metabolites produced by the body. It refers to the direct measurement of metabolites in an individual's bodily fluids, in order to predict or evaluate the metabolism of pharmaceutical compounds, and to better understand the pharmacokinetic profile of a drug. Alternatively, pharmacometabolomics can be applied to measure metabolite levels following the administration of a pharmaceutical compound, in order to monitor the effects of the compound on certain metabolic pathways(pharmacodynamics). This provides detailed mapping of drug effects on metabolism and the pathways that are implicated in mechanism of variation of response to treatment. In addition, the metabolic profile of an individual at baseline (metabotype) provides information about how individuals respond to treatment and highlights heterogeneity within a disease state. All three approaches require the quantification of metabolites found in bodily fluids and tissue, such as blood or urine, and can be used in the assessment of pharmaceutical treatment options for numerous disease states.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have application as antidepressants and in the treatment of depression and other psychiatric disorders. SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), and anorexia. There is also clinical evidence of the value of SSRIs in the treatment of the symptoms of schizophrenia and their ability to prevent cardiovascular diseases.
The term "me-too drug" or "follow-on drug" refers to a medication that is similar to a pre-existing drug, usually by making minor modifications to the prototype, reflected in slight changes in the profiles of side effects or activity, and used to treat conditions for which drugs already exist. While pharmaceutical companies have justified the development of me-toos as offering incremental improvements in efficacy, side-effects, compliance and cost, critics have questioned the increasing marketing of me-toos, their absorption of research and development resources and their impact on the innovation of new treatments.

Dehydronorketamine (DHNK), or 5,6-dehydronorketamine, is a minor metabolite of ketamine which is formed by dehydrogenation of its metabolite norketamine. Though originally considered to be inactive, DHNK has been found to act as a potent and selective negative allosteric modulator of the α7-nicotinic acetylcholine receptor (IC50 = 55 nM). For this reason, similarly to hydroxynorketamine (HNK), it has been hypothesized that DHNK may have the capacity to produce rapid antidepressant effects. However, unlike ketamine, norketamine, and HNK, DHNK has been found to be inactive in the forced swim test (FST) in mice at doses up to 50 mg/kg. DHNK is inactive at the α3β4-nicotinic acetylcholine receptor (IC50 > 100 μM) and is only very weakly active at the NMDA receptor (Ki = 38.95 μM for (S)-(+)-DHNK). It can be detected 7–10 days after a modest dose of ketamine, and because of this, is useful in drug detection assays.
A chiral switch is a chiral drug that has already approved as racemate but has been re-developed as a single enantiomer. The term chiral switching was introduced by Agranat and Caner in 1999 to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coined eutomer and distomer. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.











