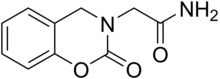
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders.

Phenelzine, sold under the brand name Nardil, among others, is a non-selective and irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class which is primarily used as an antidepressant and anxiolytic. Along with tranylcypromine and isocarboxazid, phenelzine is one of the few non-selective and irreversible MAOIs still in widespread clinical use. It is typically available in 15 mg tablets and doses usually range from 45–90 mg per day.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Tranylcypromine, sold under the brand name Parnate among others, is a monoamine oxidase inhibitor (MAOI). More specifically, tranylcypromine acts as nonselective and irreversible inhibitor of the enzyme monoamine oxidase (MAO). It is used as an antidepressant and anxiolytic agent in the clinical treatment of mood and anxiety disorders, respectively.

Tyramine, also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs).

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It is provided in the form of a capsule or tablet taken by mouth for Parkinson's disease and as a patch applied to skin for depression.

Isocarboxazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class used as an antidepressant. Along with phenelzine and tranylcypromine, it is one of only three classical MAOIs still available for clinical use in the treatment of psychiatric disorders in the United States, though it is not as commonly employed in comparison to the others.

Moclobemide, sold under the brand names Amira, Aurorix, Clobemix, Depnil and Manerix among others, is a reversible inhibitor of monoamine oxidase A (RIMA) drug primarily used to treat depression and social anxiety. It is not approved for use in the United States, but is approved in other Western countries such as Canada, the UK and Australia. It is produced by affiliates of the Hoffmann–La Roche pharmaceutical company. Initially, Aurorix was also marketed by Roche in South Africa, but was withdrawn after its patent rights expired and Cipla Medpro's Depnil and Pharma Dynamic's Clorix became available at half the cost.

Brofaromine is a reversible inhibitor of monoamine oxidase A (RIMA) discovered by Ciba-Geigy. The compound was primarily researched in the treatment of depression and anxiety but its development was dropped before it was brought to market.
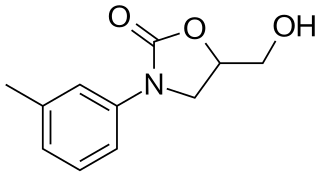
Toloxatone (Humoryl) is an antidepressant launched in 1984 in France by Sanofi Aventis for the treatment of depression. It was discontinued in 2002. It acts as a selective reversible inhibitor of MAO-A (RIMA).

Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.

Minaprine is a monoamine oxidase inhibitor antidepressant drug that was used in France for the treatment of depression until it was withdrawn from the market in 1996 because it caused convulsions.

Ciclazindol (WY-23409) is an antidepressant and anorectic drug of the tetracyclic chemical class that was developed in the mid to late 1970s, but was never marketed. It acts as a norepinephrine reuptake inhibitor, and to a lesser extent as a dopamine reuptake inhibitor. Ciclazindol has no effects on the SERT, 5-HT receptors, mACh receptors, or α-adrenergic receptors, and has only weak affinity for the H1 receptor. As suggested by its local anesthetic properties, ciclazindol may also inhibit sodium channels. It is known to block potassium channels as well.

Cimoxatone is a reversible inhibitor of MAO-A (RIMA). It has a significant food interaction–related adverse effect in combination with tyramine. It was never marketed.

Bifemelane (INN), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that is widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. Bifemelane acts as a monoamine oxidase inhibitor (MAOI) of both isoenzymes, with competitive (reversible) inhibition of MAO-A and non-competitive (irreversible) inhibition of MAO-B, and also acts (weakly) as a norepinephrine reuptake inhibitor. The drug has nootropic, neuroprotective, and antidepressant-like effects in animal models, and appears to enhance the cholinergic system in the brain.
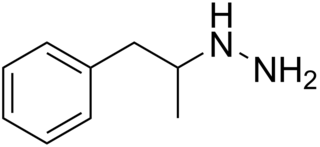
Pheniprazine is an irreversible and nonselective monoamine oxidase inhibitor (MAOI) of the hydrazine chemical class that was used as an antidepressant in the 1960s. It was also used in the treatment of angina pectoris and schizophrenia. Pheniprazine has been largely discontinued due to toxicity concerns such as jaundice, amblyopia, and optic neuritis.
Metralindole (Inkazan) is a reversible inhibitor of monoamine oxidase A (RIMA) which was investigated in Russia as a potential antidepressant. It is structurally and pharmacologically related to pirlindole.

Almoxatone (MD-780,236) is a selective and reversible inhibitor of MAO-B. It was patented as an antidepressant and antiparkinsonian agent but was never marketed.
Paraxazone is an antidepressant. It acts as a reversible inhibitor of the enzyme monoamine oxidase (MAO). It was never marketed.
Mary Lilias Christian Bernheim was a British biochemist best known for her discovery of the enzyme tyramine oxidase, which was later renamed as monoamine oxidase. Bernheim discovered the enzyme system of tyramine oxidase during her doctorate research at the University of Cambridge in 1928, and her research has been referred to as "one of the seminal discoveries in twentieth century neurobiology".