Methylation, in the chemical sciences, is the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology.

Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin.
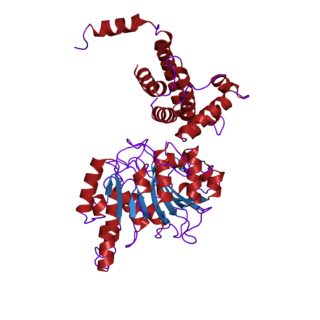
In biochemistry, the DNA methyltransferase family of enzymes catalyze the transfer of a methyl group to DNA. DNA methylation serves a wide variety of biological functions. All the known DNA methyltransferases use S-adenosyl methionine (SAM) as the methyl donor.

Histamine is an organic nitrogenous compound involved in local immune responses communication, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered in 1910, it has been considered a local hormone (autocoid) because it lacks the classic endocrine glands to secrete it; however, in recent years, histamine has been recognized as a central neurotransmitter. Histamine is involved in the inflammatory response and has a central role as a mediator of itching. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues. It consists of an imidazole ring attached to an ethylamine chain; under physiological conditions, the amino group of the side-chain is protonated.
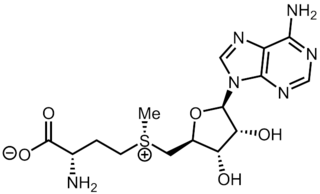
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.

The H1 receptor is a histamine receptor belonging to the family of rhodopsin-like G-protein-coupled receptors. This receptor is activated by the biogenic amine histamine. It is expressed in smooth muscles, on vascular endothelial cells, in the heart, and in the central nervous system. The H1 receptor is linked to an intracellular G-protein (Gq) that activates phospholipase C and the inositol triphosphate (IP3) signalling pathway. Antihistamines, which act on this receptor, are used as anti-allergy drugs. The crystal structure of the receptor has been determined (shown on the right/below) and used to discover new histamine H1 receptor ligands in structure-based virtual screening studies.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

D-amino acid oxidase is an enzyme with the function on a molecular level to oxidize D-amino acids to the corresponding α-keto acids, producing ammonia and hydrogen peroxide. This results in a number of physiological effects in various systems, most notably the brain. The enzyme is most active toward neutral D-amino acids, and not active toward acidic D-amino acids. One of its most important targets in mammals is D-Serine in the central nervous system. By targeting this and other D-amino acids in vertebrates, DAAO is important in detoxification. The role in microorganisms is slightly different, breaking down D-amino acids to generate energy.

Phenylethanolamine N-methyltransferase (PNMT) is an enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine (adrenaline). It is also expressed in small groups of neurons in the human brain and in selected populations of cardiomyocytes.

N-Acetylserotonin O-methyltransferase, also known as ASMT, is an enzyme which catalyzes the final reaction in melatonin biosynthesis: converting Normelatonin to melatonin. This reaction is embedded in the more general tryptophan metabolism pathway. The enzyme also catalyzes a second reaction in tryptophan metabolism: the conversion of 5-hydroxy-indoleacetate to 5-methoxy-indoleacetate. The other enzyme which catalyzes this reaction is n-acetylserotonin-o-methyltransferase-like-protein.

Diamine oxidase (DAO), also known "amine oxidase, copper-containing, 1" (AOC1), formerly called histaminase, is an enzyme involved in the metabolism, oxidation, and inactivation of histamine and other polyamines such as putrescine or spermidine. The enzyme belongs to the amine oxidase (copper-containing) (AOC) family of amine oxidase enzymes.
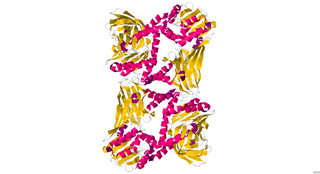
CARM1, also known as PRMT4, is an enzyme encoded by the CARM1 gene found in human beings, as well as many other mammals. It has a polypeptide (L) chain type that is 348 residues long, and is made up of alpha helices and beta sheets. Its main function includes catalyzing the transfer of a methyl group from S-Adenosyl methionine to the side chain nitrogens of arginine residues within proteins to form methylated arginine derivatives and S-Adenosyl-L-homocysteine. CARM1 is a secondary coactivator through its association with p160 family of coactivators. It is responsible for moving cells toward the inner cell mass in developing blastocysts.
Amine N-methyltransferase, also called indolethylamine N-methyltransferase, and thioether S-methyltransferase, is an enzyme that is ubiquitously present in non-neural tissues and catalyzes the N-methylation of tryptamine and structurally related compounds. More recently, it was discovered that this enzyme can also catalyze the methylation of thioether and selenoether compounds, although the physiological significance of this biotransformation is not yet known.
In enzymology, a carnosine N-methyltransferase is an enzyme that catalyzes the chemical reaction

Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression. EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27, by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function. Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.
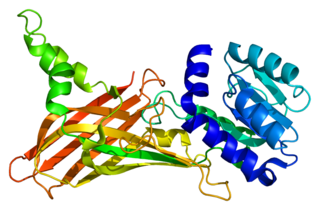
Protein arginine N-methyltransferase 1 is an enzyme that in humans is encoded by the PRMT1 gene. The HRMT1L2 gene encodes a protein arginine methyltransferase that functions as a histone methyltransferase specific for histone H4.
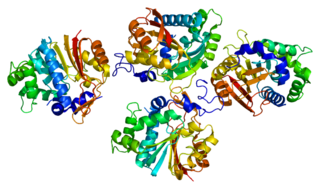
Nicotinamide N-methyltransferase (NNMT) is an enzyme that in humans is encoded by the NNMT gene. NNMT catalyzes the methylation of nicotinamide and similar compounds using the methyl donor S-adenosyl methionine (SAM-e) to produce S-adenosyl-L-homocysteine (SAH) and 1-methylnicotinamide.

Glycine N-methyltransferase is an enzyme that in humans is encoded by the GNMT gene.
Histamine intolerance is a presumed set of adverse reactions to ingested histamine in food. The mainstream theory accepts that there may exist adverse reactions to ingested histamine, but does not recognize histamine intolerance as a separate condition that can be diagnosed. There is a common suspicion that ingested histamine in persons with deficiencies in the enzymes that metabolize histamine may be responsible for various non-specific health complaints, which some individuals categorize as histamine intolerance, still, histamine intolerance is not recognized as an explicit medical condition with that name in the International Classification of Diseases (ICD) Edition 11, or any previous edition. The scientific proof that supports the idea that eating food containing histamine can cause health problems is currently limited and not consistent.

1-Methylhistamine is a metabolite of histamine.






















