
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders.
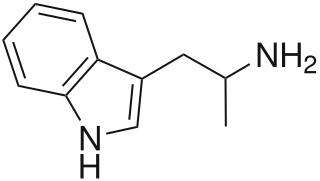
α-Methyltryptamine is a psychedelic, stimulant, and entactogen drug of the tryptamine class. It was originally developed as an antidepressant by workers at Upjohn in the 1960s, and was used briefly as an antidepressant in Russia under the trade name Indopan before being discontinued.

Tranylcypromine, sold under the brand name Parnate among others, is a monoamine oxidase inhibitor (MAOI). More specifically, tranylcypromine acts as nonselective and irreversible inhibitor of the enzyme monoamine oxidase (MAO). It is used as an antidepressant and anxiolytic agent in the clinical treatment of mood and anxiety disorders, respectively.

β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective, cognitive enhancing and anti-cancer properties.
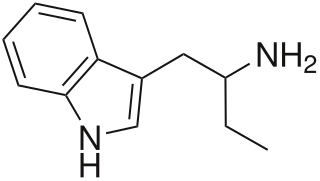
α-Ethyltryptamine, also known as etryptamine, is a psychedelic, stimulant, and entactogenic drug of the tryptamine class. It was originally developed and marketed as an antidepressant under the brand name Monase by Upjohn in the 1960s.

Several alkaloids that function as monoamine oxidase inhibitors (MAOIs) are found in the seeds of Peganum harmala, as well as tobacco leaves including harmine, harmaline, and harmalol, which are members of a group of substances with a similar chemical structure collectively known as harmala alkaloids. These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. The harmala alkaloid harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline. Harmine and harmaline are both a reversible inhibitor of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.

Nomifensine (Merital, Alival) is a norepinephrine-dopamine reuptake inhibitor, i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters. This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.

Rasagiline is an irreversible inhibitor of monoamine oxidase-B used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.
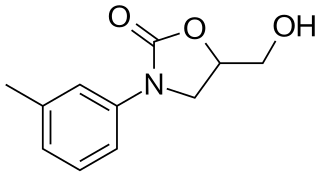
Toloxatone (Humoryl) is an antidepressant launched in 1984 in France by Sanofi Aventis for the treatment of depression. It was discontinued in 2002. It acts as a selective reversible inhibitor of MAO-A (RIMA).
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Indatraline hydrochloride is an antidepressive agent and non-selective monoamine transporter inhibitor that blocks the reuptake of dopamine, norepinephrine, and serotonin with similar efficacy to cocaine. This compound may be used to treat cocaine addictions as its effects have a slower onset and a longer duration than those of cocaine. Lu 19-005 has been shown to block the action of methamphetamine and MDMA in laboratory experiments.

N-Methylphenethylamine (NMPEA) is a naturally occurring trace amine neuromodulator in humans that is derived from the trace amine, phenethylamine (PEA). It has been detected in human urine and is produced by phenylethanolamine N-methyltransferase with phenethylamine as a substrate, which significantly increases PEA's effects. PEA breaks down into Phenylacetaldehyde which is further broken down into Phenylacetic acid by Monoamine oxidase. When this is inhibited by Monoamine oxidase inhibitors, it allows more of the PEA to be metabolized into nymphetamine (NMPEA) and not wasted on the weaker inactive metabolites.

Monoamine oxidase B, also known as MAOB, is an enzyme that in humans is encoded by the MAOB gene.

Bifemelane (INN) (Alnert, Celeport), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that is widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. Bifemelane acts as a monoamine oxidase inhibitor (MAOI) of both isoenzymes, with competitive (reversible) inhibition of MAO-A (Ki = 4.20 μM) (making it a reversible inhibitor of monoamine oxidase A (RIMA)) and non-competitive (irreversible) inhibition of MAO-B (Ki = 46.0 μM), and also acts (weakly) as a norepinephrine reuptake inhibitor. The drug has nootropic, neuroprotective, and antidepressant-like effects in animal models, and appears to enhance the cholinergic system in the brain.
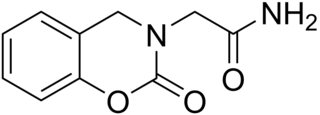
Caroxazone is an antidepressant which was formerly used for the treatment of depression but is now no longer marketed. It acts as a reversible monoamine oxidase inhibitor (RIMA) of both MAO-A and MAO-B subtypes, with five-fold preference for the latter.

Almoxatone (MD-780,236) is a selective and reversible inhibitor of MAO-B. It was patented as an antidepressant and antiparkinsonian agent but was never marketed.

7-Methyl-α-ethyltryptamine (7-Me-αET) is a tryptamine derivative related to α-ethyltryptamine (αET). It was discovered by a team at Upjohn in the early 1960s. It has similar pharmacological effects to αET, but is both 3-4 times more potent as a serotonin releasing agent, and 10 times more potent as a monoamine oxidase inhibitor, making it potentially hazardous as this pharmacological profile is shared with drugs such as PMA and 4-MTA, which are known to be dangerous in humans when used at high doses.
Tetrindole was a drug candidate that functions by reversibly inhibiting monoamine oxidase A; it was first synthesized in Moscow in the early 1990s. Tetrindole is similar in its chemical structure to pirlindole (Pyrazidol), and metralindole.

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

Eprobemide (INN) is a pharmaceutical drug that was used as an antidepressant in Russia. It is a non-competitive reversible inhibitor of monoamine oxidase A that exhibits selective action on serotonin deamination. Eprobemide differs from moclobemide only in the linker that connects the morpholine fragment with the chlorobenzamide — moclobemide has two carbon atoms while eprobemide has three. Its registration was cancelled on December 30, 2003.



















