
Monoamine transporters (MATs) are proteins that function as integral plasma-membrane transporters to regulate concentrations of extracellular monoamine neurotransmitters. The three major classes are serotonin transporters (SERTs), dopamine transporters (DATs), and norepinephrine transporters (NETs) and are responsible for the reuptake of their associated amine neurotransmitters. MATs are located just outside the synaptic cleft (peri-synaptically), transporting monoamine transmitter overflow from the synaptic cleft back to the cytoplasm of the pre-synaptic neuron. MAT regulation generally occurs through protein phosphorylation and post-translational modification. Due to their significance in neuronal signaling, MATs are commonly associated with drugs used to treat mental disorders as well as recreational drugs. Compounds targeting MATs range from medications such as the wide variety of tricyclic antidepressants, selective serotonin reuptake inhibitors such as fluoxetine (Prozac) to stimulant medications such as methylphenidate (Ritalin) and amphetamine in its many forms and derivatives methamphetamine (Desoxyn) and lisdexamfetamine (Vyvanse). Furthermore, drugs such as MDMA and natural alkaloids such as cocaine exert their effects in part by their interaction with MATs, by blocking the transporters from mopping up dopamine, serotonin, and other neurotransmitters from the synapse.
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission.
The vesicular monoamine transporter (VMAT) is a transport protein integrated into the membranes of synaptic vesicles of presynaptic neurons. It transports monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses, as chemical messages to postsynaptic neurons. VMATs utilize a proton gradient generated by V-ATPases in vesicle membranes to power monoamine import.

The solute carrier family 18 member 2 (SLC18A2) also known as vesicular monoamine transporter 2 (VMAT2) is a protein that in humans is encoded by the SLC18A2 gene. SLC18A2 is an integral membrane protein that transports monoamines—particularly neurotransmitters such as dopamine, norepinephrine, serotonin, and histamine—from cellular cytosol into synaptic vesicles. In nigrostriatal pathway and mesolimbic pathway dopamine-releasing neurons, SLC18A2 function is also necessary for the vesicular release of the neurotransmitter GABA.

Vanoxerine is a piperazine derivative which is a potent and selective dopamine reuptake inhibitor (DRI). Vanoxerine binds to the target site on the dopamine transporter (DAT) ~ 50 times more strongly than cocaine, but simultaneously inhibits the release of dopamine. This combined effect only slightly elevates dopamine levels, giving vanoxerine only mild stimulant effects. Vanoxerine has also been observed to be a potent blocker of the IKr (hERG) channel. Vanoxerine also binds with nanomolar affinity to the serotonin transporter.

Phenyltropanes (PTs) were originally developed to reduce cocaine addiction and dependency. In general these compounds act as inhibitors of the plasmalemmal monoamine reuptake transporters. This research has spanned beyond the last couple decades, and has picked up its pace in recent times, creating numerous phenyltropanes as research into cocaine analogues garners interest to treat addiction.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Reuptake inhibitors (RIs) are a type of reuptake modulators. It is a drug that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.
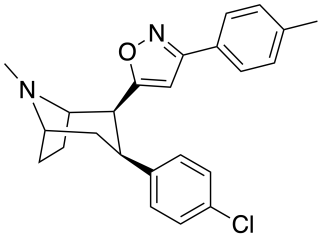
RTI(-4229)-336, is a phenyltropane derivative which acts as a potent and selective dopamine reuptake inhibitor and stimulant drug. It binds to the dopamine transporter with around 20x the affinity of cocaine, however it produces relatively mild stimulant effects, with a slow onset and long duration of action. These characteristics make it a potential candidate for treatment of cocaine addiction, as a possible substitute drug analogous to how methadone is used for treating heroin abuse. RTI-336 fully substitutes for cocaine in addicted monkeys and supports self-administration, and significantly reduces rates of cocaine use, especially when combined with SSRIs, and research is ongoing to determine whether it could be a viable substitute drug in human cocaine addicts.

GBR-12935 is a piperazine derivative which is a potent and selective dopamine reuptake inhibitor. It was originally developed in its 3H radiolabelled form for the purpose of mapping the distribution of dopaminergic neurons in the brain by selective labelling of dopamine transporter proteins. This has led to potential clinical uses in the diagnosis of Parkinson's disease, although selective radioligands such as Ioflupane (¹²³I) are now available for this application. GBR-12935 is now widely used in animal research into Parkinson's disease and the dopamine pathways in the brain.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.

Benocyclidine, also known as benzothiophenylcyclohexylpiperidine (BTCP), is a psychoactive recreational drug of the arylcyclohexylamine class which is related to phencyclidine (PCP). It was first described in a patent application naming Marc Caron and colleagues at Duke University in 1997.
A dopamine releasing agent (DRA) is a type of drug which induces the release of dopamine in the body and/or brain. No selective DRAs are currently known. Many releasing agents of both dopamine and norepinephrine and of serotonin, norepinephrine, and dopamine are known, however. Serotonin–dopamine releasing agents are much rarer and are not selective for monoamine release. Examples of NDRAs include amphetamine and methamphetamine, and an example of an SNDRA is MDMA. The most selective dopamine releaser is 4-methylaminorex, but it also has considerable activity as a norepinephrine releaser. These drugs are frequently used for recreational purposes and encountered as drugs of abuse.
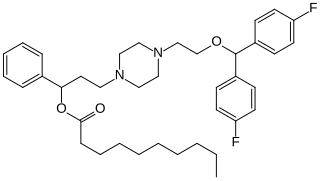
DBL-583 is a selective dopamine reuptake inhibitor of the piperazine chemical class. It is the decanoate ester of a hydroxy vanoxerine. DBL-583 breaks down very slowly in the body, lasting for up to a month after a single injection.

The plasma membrane monoamine transporter (PMAT) is a low-affinity monoamine transporter protein which in humans is encoded by the SLC29A4 gene. It is known alternatively as the human equilibrative nucleoside transporter-4 (hENT4). It was discovered in 2004 and has been identified as a potential alternate target for treating various conditions.
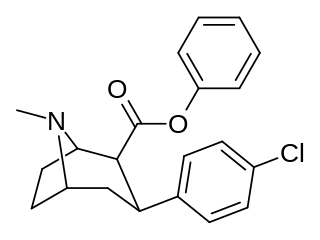
RTI(-4229)-113 is a stimulant drug which acts as a potent and fully selective dopamine reuptake inhibitor (DRI). It has been suggested as a possible substitute drug for the treatment of cocaine addiction. "RTI-113 has properties that make it an ideal medication for cocaine abusers, such as an equivalent efficacy, a higher potency, and a longer duration of action as compared to cocaine." Replacing the methyl ester in RTI-31 with a phenyl ester makes the resultant RTI-113 fully DAT specific. RTI-113 is a particularly relevant phenyltropane cocaine analog that has been tested on squirrel monkeys. RTI-113 has also been tested against cocaine in self-administration studies for DAT occupancy by PET on awake rhesus monkeys. The efficacy of cocaine analogs to elicit self-administration is closely related to the rate at which they are administered. Slower onset of action analogs are less likely to function as positive reinforcers than analogues that have a faster rate of onset.
A monoamine reuptake inhibitor (MRI) is a drug that acts as a reuptake inhibitor of one or more of the three major monoamine neurotransmitters serotonin, norepinephrine, and dopamine by blocking the action of one or more of the respective monoamine transporters (MATs), which include the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT). This in turn results in an increase in the synaptic concentrations of one or more of these neurotransmitters and therefore an increase in monoaminergic neurotransmission.

GBR-13069 is a psychostimulant and selective dopamine reuptake inhibitor.

Decynium-22 is a cationic derivative of quinoline, and a potent inhibitor of the plasma membrane monoamine transporter (PMAT), as well as all members of the organic cation transporter (OCT) family in both human and rat cells. However, it has little effect on high affinity monoamine transporters such as the dopamine transporter and norepinephrine transporter.
An excitatory amino acid reuptake inhibitor (EAARI) is a type of drug which inhibits the reuptake of the excitatory neurotransmitters glutamate and aspartate by blocking one or more of the excitatory amino acid transporters (EAATs).















