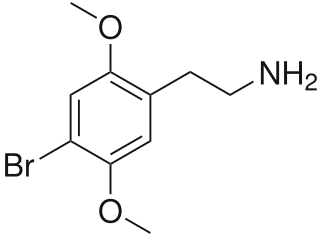
2C-B (4-bromo-2,5-dimethoxyphenethylamine) is a synthetic psychedelic drug of the 2C family, initially synthesized by Alexander Shulgin in 1974 and commonly used as a recreational drug. There is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant, and hallucinogen, and less commonly as an entactogen, and empathogen.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.

Dimethoxybromoamphetamine (DOB), also known as brolamfetamine (INN) and bromo-DMA, is a psychedelic drug and substituted amphetamine of the phenethylamine class of compounds. DOB was first synthesized by Alexander Shulgin in 1967. Its synthesis and effects are documented in Shulgin's book PiHKAL: A Chemical Love Story.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Bromo-DragonFLY is a substance related to the phenethylamine family. It acts as a potent full agonist for the 5-HT2A receptor.

Benzylpiperazine (BZP) is a substance often used as a recreational drug and is known to have euphoriant and stimulant properties. Several studies conducted between 2000 and 2011 found that the effects of BZP are similar to amphetamine, although BZP's dosage is roughly 10 times higher by weight.
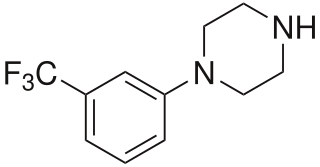
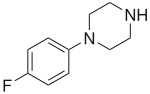
3-Trifluoromethylphenylpiperazine (TFMPP) is a recreational drug of the phenylpiperazine chemical class and is a substituted piperazine. Usually in combination with benzylpiperazine (BZP) and other analogues, it is sold as an alternative to the illicit drug MDMA ("Ecstasy").
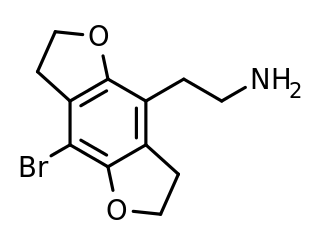
2C-B-FLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron Monte, Professor of Chemistry at UW-La Crosse.

Party pills, also known as "herbal highs", "pep pills" "dance pills" and "natural power", is a colloquialism for a type of recreational drug whose main ingredient was originally benzylpiperazine (BZP), but has expanded to a wide range of compounds with a variety of effects. BZP is banned in several countries, including the USA, Republic of Ireland, Australia and New Zealand, but is available on a more or less restricted basis in many jurisdictions. A range of other piperazine derivatives have also been sold as ingredients in party pills, and many of these branded "proprietary blends" have subsequently been sold in countries around the world.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.

para-Methoxyphenylpiperazine is a piperazine derivative with stimulant effects which has been sold as an ingredient in "Party pills", initially in New Zealand and subsequently in other countries around the world.

Niaprazine (INN) is a sedative-hypnotic drug of the phenylpiperazine group. It has been used in the treatment of sleep disturbances since the early 1970s in several European countries including France, Italy, and Luxembourg. It is commonly used with children and adolescents on account of its favorable safety and tolerability profile and lack of abuse potential.

Methylbenzylpiperazine is a stimulant drug which is a derivative of benzylpiperazine. MBZP has been sold as an ingredient in legal recreational drugs known as "party pills", initially in New Zealand and subsequently in other countries around the world.

25I-NBOMe is a novel synthetic psychoactive substance with strong hallucinogenic properties, synthesized in 2003 for research purposes. Since 2010, it has circulated in the recreational drug scene, often misrepresented as LSD. The recreational usage of 25I is associated with severe intoxication and deaths in humans.
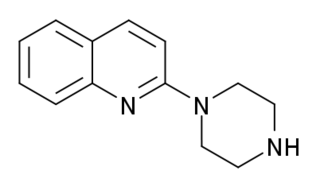
Quipazine is a serotonergic drug of the piperazine group which is used in scientific research. It was originally intended as an antidepressant but never developed for medical use.

Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor. Osemozotan has antidepressant, anxiolytic, antiobsessional, serenic, and analgesic effects in animal studies, and is used to investigate the role of 5-HT1A receptors in modulating the release of dopamine and serotonin in the brain, and their involvement in addiction to abused stimulants such as cocaine and methamphetamine.
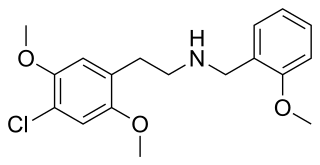
25C-NBOMe is a psychedelic drug and derivative of the psychedelic phenethylamine 2C-C. 25C-NBOMe appeared on online vendor sites in 2010 but was not reported in the literature until 2011. It acts as a potent agonist of the 5-HT2A receptor, and has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET). Multiple deaths have occurred from usage of 25C-NBOMe due to the ease of accidental overdose. The long-term toxic effects of the drug have not been researched.

NBOMe-mescaline or mescaline-NBOMe is a synthetic substituted phenethylamine. It is a partial agonist of serotonin receptors with a 5-HT2A pKi originally reported as 7.3, though more modern techniques assayed it as 140nM at 5-HT2A and 640nM at 5-HT2C, making it one of the least potent compounds among the N-benzyl phenethylamines.