
The drug combination fenfluramine/phentermine, usually called fen-phen, was an anti-obesity treatment in the early 1990s that utilized two anorectics. Fenfluramine was marketed by American Home Products as Pondimin, but was shown to cause potentially fatal pulmonary hypertension and heart valve problems, which eventually led to its withdrawal in 1997 and legal damages of over $13 billion. Phentermine was not shown to have harmful effects.

Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome. It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications. Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine under the brand name Fen-Phen among others.

4-Methylaminorex is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories. It is also known by its street name "U4Euh" ("Euphoria"). It is banned in many countries as a stimulant.

Naphthylaminopropane (PAL-287) is an experimental drug under investigation as of 2007 for the treatment of alcohol and stimulant addiction.

Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family that behaves as a serotonin and norepinephrine releasing agent and potent 5-HT2A, 5-HT2B, and 5-HT2C agonist. The action of norfenfluramine on 5-HT2B receptors on heart valves leads to a characteristic pattern of heart failure following proliferation of cardiac fibroblasts on the tricuspid valve, known as cardiac fibrosis. This side effect led to the withdrawal of fenfluramine as an anorectic agent worldwide, and to the withdrawal of benfluorex in Europe, as both fenfluramine and benfluorex form norfenfluramine as an active metabolite. It is a human TAAR1 agonist.
In pharmacology, an indirect agonist or indirect-acting agonist is a substance that enhances the release or action of an endogenous neurotransmitter but has no specific agonist activity at the neurotransmitter receptor itself. Indirect agonists work through varying mechanisms to achieve their effects, including transporter blockade, induction of transmitter release, and inhibition of transmitter breakdown.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher neurotoxicity, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite. It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.
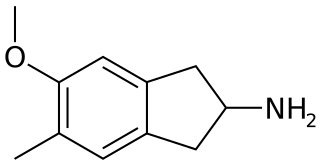
5-Methoxy-6-methyl-2-aminoindane (MMAI) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) and produces entactogen effects in humans. It has been sold as a designer drug and research chemical online since 2010.
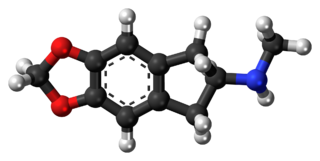
5,6-Methylenedioxy-N-methyl-2-aminoindane (MDMAI), is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in animals and a putative entactogen in humans.

5-Iodo-2-aminoindane (5-IAI) is a drug which acts as a releasing agent of serotonin, norepinephrine, and dopamine. It was developed in the 1990s by a team led by David E. Nichols at Purdue University. 5-IAI fully substitutes for MDMA in rodents and is a putative entactogen in humans. Unlike related aminoindane derivatives like MDAI and MMAI, 5-IAI causes some serotonergic neurotoxicity in rats, but is substantially less toxic than its corresponding amphetamine homologue pIA, with the damage observed barely reaching statistical significance.

2-Aminoindane (2-AI) is a research chemical with applications in neurologic disorders and psychotherapy that has also been sold as a designer drug. It acts as a selective substrate for NET and DAT.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.

5-Trifluoromethyl-2-aminoindane (TAI) is a psychoactive drug and research chemical with putative entactogenic effects. It functions as a selective serotonin releasing agent (SSRA). TAI is the aminoindane analogue of norfenfluramine and is approximately 50% as neurotoxic in comparison.

Levofenfluramine (INN), or (−)-3-trifluoromethyl-N-ethylamphetamine, also known as (−)-fenfluramine or (R)-fenfluramine, is a drug of the amphetamine family that, itself (i.e., in enantiopure form), was never marketed. It is the levorotatory enantiomer of fenfluramine, the racemic form of the compound, whereas the dextrorotatory enantiomer is dexfenfluramine. Both fenfluramine and dexfenfluramine are anorectic agents that have been used clinically in the treatment of obesity (and hence, levofenfluramine has been as well since it is a component of fenfluramine). However, they have since been discontinued due to reports of causing cardiovascular conditions such as valvular heart disease and pulmonary hypertension, adverse effects that are likely to be caused by excessive stimulation of 5-HT2B receptors expressed on heart valves.

25D-NBOMe is a derivative of the phenethylamine derived hallucinogen 2C-D. It acts in a similar manner to related compounds such as 25I-NBOMe, which is a potent agonist at the 5HT2A receptor. 25D-NBOMe has been sold as a street drug since 2010 and produces similar effects in humans to related compounds such as 25I-NBOMe and 25C-NBOMe. It was banned as a Temporary Class Drug in the UK on 10 June 2013 after concerns about its recreational use.

25E-NBOMe is a derivative of the phenethylamine 2C-E. It acts in a similar manner to related compounds such as 25I-NBOMe, which are potent agonists at the 5HT2A receptor. 25E-NBOMe has been sold as a drug and produces similar effects in humans to related compounds such as 25I-NBOMe and 25C-NBOMe.

25G-NBOMe (NBOMe-2C-G) is a derivative of the phenethylamine hallucinogen 2C-G, which acts as a highly potent agonist for the human 5-HT2A receptor.

25H-NBOMe (NBOMe-2C-H) is a derivative of the phenethylamine hallucinogen 2C-H, which acts as a highly potent full agonist for the human 5-HT2A receptor.

25iP-NBOMe is a derivative of the phenethylamine hallucinogen 2C-iP, which acts as a highly potent agonist for the human 5-HT2A receptor.


















