
3,4-Methylenedioxyamphetamine (MDA), sometimes referred to as sass, is an empathogen-entactogen, stimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.

Amoxapine, sold under the brand name Asendin among others, is a tricyclic antidepressant (TCA). It is the N-demethylated metabolite of loxapine. Amoxapine first received marketing approval in the United States in 1980, approximately 10 to 20 years after most of the other TCAs were introduced in the United States.

Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood. In 1885, cocaethylene was first synthesized, and in 1979, cocaethylene's side effects were discovered.

Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH). In the United States, aminorex is a Schedule I controlled substance.

Etilamfetamine, also known as N-ethylamphetamine and formerly sold under the brand names Apetinil and Adiparthrol, is a stimulant drug of the amphetamine family. It was invented in the early 20th century and was subsequently used as an anorectic or appetite suppressant in the 1950s, but was not as commonly used as other amphetamines such as amphetamine, methamphetamine, and benzphetamine, and was largely discontinued once newer drugs such as phenmetrazine were introduced.

11-Hydroxy-Δ9-tetrahydrocannabinol, usually referred to as 11-hydroxy-THC is the main active metabolite of tetrahydrocannabinol (THC), which is formed in the body after Δ9-THC is consumed.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with CYP2D6 inactivating mutations.
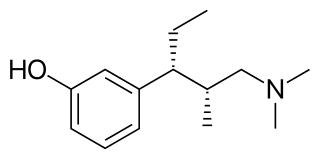
Tapentadol, sold under the brand names Nucynta and Palexia among others, is a synthetic opioid analgesic of the benzenoid class with a dual mode of action as a highly selective full agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Tapentadol is used medically for the treatment of moderate to severe pain. It is addictive, a commonly abused drug, and poses a high risk of physical and/or mental dependence.

Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the TAAR1 gene.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

Medifoxamine, previously sold under the brand names Clédial and Gerdaxyl, is an atypical antidepressant with additional anxiolytic properties acting via dopaminergic and serotonergic mechanisms which was formerly marketed in France and Spain, as well as Morocco. The drug was first introduced in France sometime around 1990. It was withdrawn from the market in 1999 (Morocco) and 2000 (France) following incidences of hepatotoxicity.
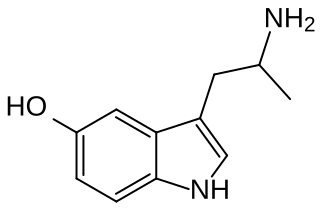
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.
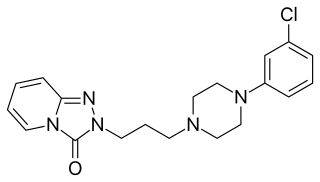
Serotonin antagonist and reuptake inhibitors (SARIs) are a class of drugs used mainly as antidepressants, but also as anxiolytics and hypnotics. They act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine, and/or dopamine. Additionally, most also antagonize α1-adrenergic receptors. The majority of the currently marketed SARIs belong to the phenylpiperazine class of compounds.

Ephenidine is a dissociative anesthetic that has been sold online as a designer drug. It is illegal in some countries as a structural isomer of the banned opioid drug lefetamine, but has been sold in countries where it is not yet banned.

Solriamfetol, sold under the brand name Sunosi, is a wakefulness-promoting medication used in the treatment of excessive sleepiness related to narcolepsy and sleep apnea. It is taken by mouth.
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of chemical compounds that are used to reverse adverse effects caused by opioids interacting with receptors outside the central nervous system (CNS), mainly those located in the gastrointestinal tract. PAMORAs are designed to specifically inhibit certain opioid receptors in the gastrointestinal tract and with limited ability to cross the blood–brain barrier. Therefore, PAMORAs do not affect the analgesic effects of opioids within the central nervous system.

Threohydrobupropion is a substituted amphetamine derivative—specifically a β-hydroxyamphetamine—and a major active metabolite of the antidepressant drug bupropion (Wellbutrin). Bupropion is a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator, with its metabolites contributing substantially to its activities.

(R)-1-Aminoindane ((R)-1-AI; developmental code name TVP-136 or TV-136), or (R)-1-aminoindan, is the major metabolite of the selective MAO-B inhibitor and antiparkinsonian agent rasagiline ((R)-N-propargyl-1-aminoindane). In contrast to rasagiline, it lacks significant monoamine oxidase inhibition. In addition, unlike selegiline and its amphetamine metabolites, it lacks monoamine reuptake-inhibiting and -releasing activities and associated amphetamine-like psychostimulant effects. However, (R)-1-aminoindane retains neuroprotective effects and certain other activities.

1-Aminoindane (1-AI), also known as 1-aminoindan, 1-indanylamine, or 1-indanamine, is an aminoindane. It is a positional isomer of 2-aminoindane. A variety of notable derivatives of 1- and 2-aminoindane are known. The (R)-enantiomer of 1-aminoindan, (R)-1-aminoindan, is pharmacologically active and is an active metabolite of the antiparkinsonian agent rasagiline.



















