
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

3,4-Methylenedioxyamphetamine (MDA), sometimes referred to as sass, is an empathogen-entactogen, stimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
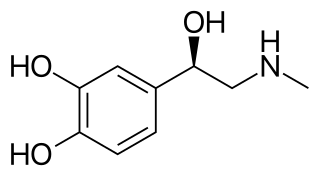
Sympathomimetic drugs are stimulant compounds which mimic the effects of endogenous agonists of the sympathetic nervous system. Examples of sympathomimetic effects include increases in heart rate, force of cardiac contraction, and blood pressure. The primary endogenous agonists of the sympathetic nervous system are the catecholamines, which function as both neurotransmitters and hormones. Sympathomimetic drugs are used to treat cardiac arrest and low blood pressure, or even delay premature labor, among other things.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
Thailand's Psychotropic Substances Act is a law designed to regulate certain mind-altering drugs. According to the Office of the Narcotics Control Board, "The Act directly resulted from the Convention on Psychotropic Substances 1971 of which Thailand is a party." The Act divides psychotropic drugs into four Schedules. Offenses involving Schedule I and II drugs carry heavier penalties than those involving Schedule III and IV drugs. Note that this statute does not regulate most opioids, cocaine, or some amphetamines. The vast majority of narcotic painkillers, along with cocaine and most amphetamines are regulated under the Narcotics Act.

The Aza-Diels–Alder reaction is a modification of the Diels–Alder reaction wherein a nitrogen replaces sp2 carbon. The nitrogen atom can be part of the diene or the dienophile.

Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Etonitazene, also known as EA-4941 or CS-4640, is a benzimidazole opioid, first reported in 1957, that has been shown to have approximately 1,000 to 1,500 times the potency of morphine in animals.
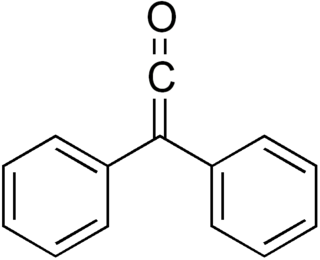
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.

1-Phenyl-2-nitropropene, or simply phenyl-2-nitropropene, or P2NP, as it is commonly referred to, is a chemical compound from the aromatic group of compounds, with the formula C9H9NO2. It is a light-yellow crystalline solid with a distinct smell. Phenyl-2-nitropropene is used in the pharmaceutical industry to manufacture the drug Adderall, an amphetamine mixture used to treat ADHD and narcolepsy. P2NP and other similar nitrostyrenes are also employed in the clandestine manufacture of drugs of the amphetamine class, and are listed as drug precursors in many countries.
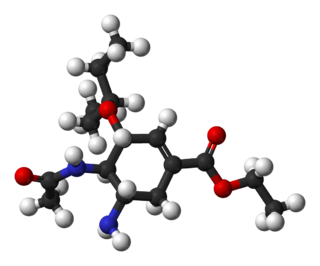
Oseltamivir total synthesis concerns the total synthesis of the anti-influenza drug oseltamivir marketed by Hoffmann-La Roche under the trade name Tamiflu. Its commercial production starts from the biomolecule shikimic acid harvested from Chinese star anise and from recombinant E. coli. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.

Naphthylaminopropane, also known as naphthylisopropylamine (NIPA), is an experimental drug that was under investigation for the treatment of alcohol and stimulant addiction.

Tilidine, sold under the brand name Valoron among others, is a synthetic opioid analgesic, used mainly in Belgium, Bulgaria, Germany, Albania, Luxembourg, South Africa, and Switzerland for the treatment of moderate to severe pain, both acute and chronic. Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after administration.

A norepinephrine–dopamine reuptake inhibitor (NDRI) is a drug used for the treatment of clinical depression, attention deficit hyperactivity disorder (ADHD), narcolepsy, and the management of Parkinson's disease. The drug acts as a reuptake inhibitor for the neurotransmitters norepinephrine and dopamine by blocking the action of the norepinephrine transporter (NET) and the dopamine transporter (DAT), respectively. This in turn leads to increased extracellular concentrations of both norepinephrine and dopamine and, therefore, an increase in adrenergic and dopaminergic neurotransmission.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective reactions.
The retro-Diels–Alder reaction is the reverse of the Diels–Alder (DA) reaction, a [4+2] cycloelimination. It involves the formation of a diene and dienophile from a cyclohexene. It can be accomplished spontaneously with heat, or with acid or base mediation.

Substituted tryptamines, or simply tryptamines, also known as serotonin analogues (i.e., 5-hydroxytryptamine analogues), are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

o-Phenyl-3-iodotyramine (o-PIT) is a drug which acts as a selective agonist for the trace amine-associated receptor 1 (TAAR1). It has reasonable selectivity for TAAR1 but relatively low potency, and is rapidly metabolised in vivo, making it less useful for research than newer ligands such as RO5166017. Its EC50Tooltip half-maximal effective concentration values have been reported to be 35 nM for the mouse TAAR1, 2.4 nM at the rat TAAR1, and 9.5 nM at the human TAAR1.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.

















