
Antidepressants are a class of medications used to treat major depressive disorder, anxiety disorders, chronic pain, and addiction.
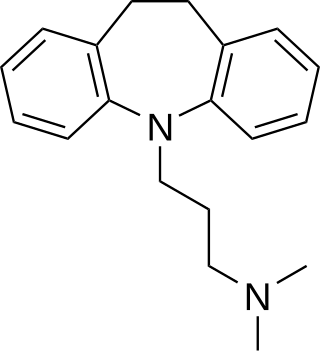
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants, which is important for the management of depression. They are second-line drugs next to SSRIs. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.
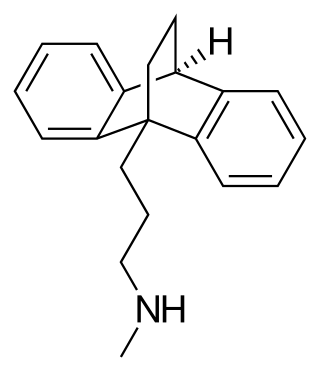
Maprotiline, sold under the brand name Ludiomil among others, is a tetracyclic antidepressant (TeCA) that is used in the treatment of depression. It may alternatively be classified as a tricyclic antidepressant (TCA), specifically a secondary amine. In terms of its chemistry and pharmacology, maprotiline is closely related to other secondary amine TCAs like nortriptyline and protriptyline, and has similar effects to them.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.

Tranylcypromine, sold under the brand name Parnate among others, is a monoamine oxidase inhibitor (MAOI). More specifically, tranylcypromine acts as nonselective and irreversible inhibitor of the enzyme monoamine oxidase (MAO). It is used as an antidepressant and anxiolytic agent in the clinical treatment of mood and anxiety disorders, respectively.

Amitriptyline, sold under the brand name Elavil among others, is a tricyclic antidepressant primarily used to treat major depressive disorder, a variety of pain syndromes such as neuropathic pain, fibromyalgia, migraine and tension headaches. Due to the frequency and prominence of side effects, amitriptyline is generally considered a second-line therapy for these indications.

Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant medications used to treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the selective serotonin reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (NRIs), which act upon single neurotransmitters.

Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) mainly used in the treatment of depression. It is also effective in treating anxiety and panic disorder. Imipramine may also be used off-label for nocturnal enuresis and chronic pain. Imipramine is taken by mouth.

Clomipramine, sold under the brand name Anafranil among others, is a tricyclic antidepressant (TCA). It is used for the treatment of obsessive–compulsive disorder, panic disorder, major depressive disorder, and chronic pain. It may increase the risk of suicide in those under the age of 25. It is primarily taken by mouth. It has also been used to treat premature ejaculation.

Nortriptyline, sold under the brand name Pamelor, among others, is a medication used to treat depression. This medicine is also sometimes used for neuropathic pain, attention deficit hyperactivity disorder (ADHD), smoking cessation and anxiety. As with many antidepressants, its use for young people with depression and other psychiatric disorders may be limited due to increased suicidality in the 18-24 population initiating treatment. Nortriptyline is a less preferred treatment for ADHD and stopping smoking. It is taken by mouth.

Doxepin is a medication belonging to the tricyclic antidepressant (TCA) class of drugs used to treat major depressive disorder, anxiety disorders, chronic hives, and insomnia. For hives it is a less preferred alternative to antihistamines. It has a mild to moderate benefit for sleeping problems. It is used as a cream for itchiness due to atopic dermatitis or lichen simplex chronicus.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression. Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs. It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.
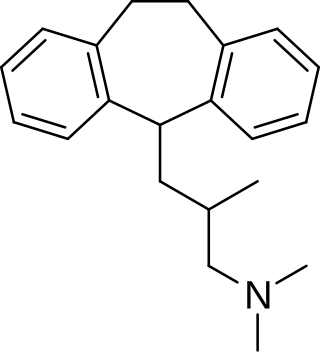
Butriptyline, sold under the brand name Evadyne among others, is a tricyclic antidepressant (TCA) that has been used in the United Kingdom and several other European countries for the treatment of depression but appears to no longer be marketed. Along with trimipramine, iprindole, and amoxapine, it has been described as an "atypical" or "second-generation" TCA due to its relatively late introduction and atypical pharmacology. It was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.
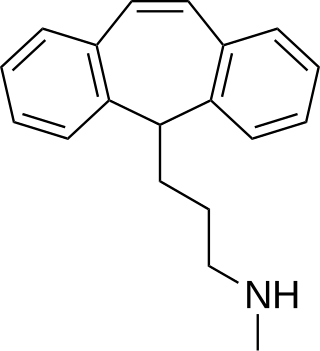
Protriptyline, sold under the brand name Vivactil among others, is a tricyclic antidepressant (TCA), specifically a secondary amine, indicated for the treatment of depression and attention-deficit hyperactivity disorder (ADHD). Uniquely among most of the TCAs, protriptyline tends to be energizing instead of sedating, and is sometimes used for narcolepsy to achieve a wakefulness-promoting effect.
A serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), also known as a triple reuptake inhibitor (TRI), is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. It does this by concomitantly inhibiting the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT), respectively. Inhibition of the reuptake of these neurotransmitters increases their extracellular concentrations and, therefore, results in an increase in serotonergic, adrenergic, and dopaminergic neurotransmission. The naturally-occurring and potent SNDRI cocaine is widely used recreationally and often illegally for the euphoric effects it produces.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.

A reuptake inhibitor (RI) is a type of drug known as a reuptake modulator that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Tandamine is a selective norepinephrine reuptake inhibitor with a tricyclic structure. It was developed in the 1970s as an antidepressant but was never commercialized. Tandamine is analogous to pirandamine, which, instead, acts as a selective serotonin reuptake inhibitor (SSRI).
Tampramine (AHR-9,377) is a tricyclic antidepressant (TCA) which was developed in the 1980s but was never marketed. Despite being a TCA, it acts as a selective norepinephrine reuptake inhibitor and has negligible affinity for adrenergic, histaminergic, and muscarinic receptors. It was found to be effective in the forced swim test (FST) model of depression in animal studies but is not known to have ever been trialed in humans.


















