
Lysergic acid diethylamide (LSD), also known colloquially as acid, is a hallucinogenic drug. Effects typically include altered thoughts, feelings, and awareness of one's surroundings. Many users have visual or auditory hallucinations. Dilated pupils, increased blood pressure, and increased body temperature are typical. Effects typically begin within half an hour and can last for up to 20 hours. It is used mainly as a recreational drug or for spiritual reasons.

Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
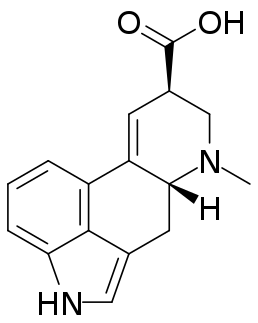
Lysergic acid, also known as D-lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and found in the seeds of Turbina corymbosa (ololiuhqui), Argyreia nervosa, and Ipomoea tricolor. Amides of lysergic acid, lysergamides, are widely used as pharmaceuticals and as psychedelic drugs (LSD). Lysergic acid received its name as it was a product of the lysis of various ergot alkaloids.

Amides of lysergic acid are collectively known as lysergamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors.
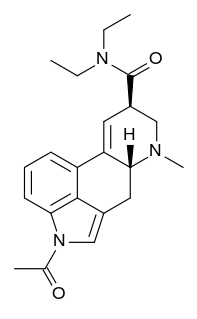
ALD-52, also known as 1-acetyl-LSD, is a chemical analogue of lysergic acid diethylamide (LSD). It was originally discovered by Albert Hofmann but was not widely studied until the rise in popularity of psychedelics in the 1960s.

Methylergometrine, also known as methylergonovine and sold under the brand name Methergine, is a medication of the ergoline and lysergamide groups which is used as an oxytocic in obstetrics and in the treatment of migraine. It reportedly produces psychedelic effects similar to those of lysergic acid diethylamide (LSD) at high doses.
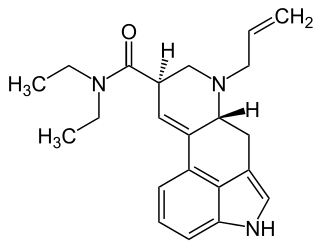
AL-LAD, also known as 6-allyl-6-nor-LSD, is a psychedelic drug and an analog of lysergic acid diethylamide (LSD). It is described by Alexander Shulgin in the book TiHKAL. It is synthesized starting from nor-LSD as a precursor, using allyl bromide as a reactant.

ETH-LAD, 6-ethyl-6-nor-lysergic acid diethylamide is an analogue of LSD. Its human psychopharmacology was first described by Alexander Shulgin in the book TiHKAL. ETH-LAD is a psychedelic drug similar to LSD, and is slightly more potent than LSD itself, with an active dose reported at between 20 and 150 micrograms. ETH-LAD has subtly different effects to LSD, described as less demanding.

PRO-LAD is an analogue of LSD. It is described by Alexander Shulgin in the book TiHKAL. PRO-LAD is a psychedelic drug similar to LSD, and is around as potent as LSD itself with an active dose reported at between 100 and 200 micrograms.

BU-LAD, also known as 6-butyl-6-nor-lysergic acid diethylamide, is an analogue of LSD first made by Alexander Shulgin and reported in the book TiHKAL. BU-LAD is a psychedelic drug similar to LSD, but is significantly less potent than LSD, with a dose of 500 micrograms producing only mild effects.

N,N-Dimethyllysergamide or N,N-dimethyl-D-lysergamide (DAM-57) is a derivative of ergine. There has been a single report of observing N,N-dimethyl-D-lysergamide in the illicit drug market. This compound did induce autonomic disturbances at oral levels of some ten times the dosage required for LSD, presumably in the high hundreds of micrograms. There is some disagreement as to whether there were psychic changes observed.

Lysergic acid 2,4-dimethylazetidide is an analog of LSD developed by the team led by David E. Nichols at Purdue University. It was developed as a rigid analog of LSD with the diethylamide group constrained into an azetidine ring in order to map the binding site at the 5-HT2A receptor. There are three possible stereoisomers around the azetidine ring, with the (S,S)-(+) isomer being the most active, slightly more potent than LSD itself in drug discrimination tests using trained rats.
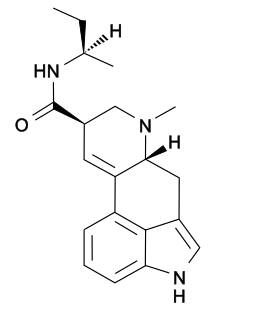
Lysergic acid 2-butyl amide (2-Butyllysergamide, LSB) is an analogue of LSD originally developed by Richard Pioch at Eli Lilly in the 1950s, but mostly publicised through research conducted by the team led by David E. Nichols at Purdue University. It is a structural isomer of LSD, with the two ethyl groups on the amide nitrogen having been replaced by a single sec-butyl group, joined at the 2-position. It is one of the few lysergamide derivatives to exceed the potency of LSD in animal drug discrimination assays, with the (R) isomer having an ED50 of 33nmol/kg for producing drug-appropriate responding, vs 48nmol/kg for LSD itself. The corresponding (R)-2-pentyl analogue has higher binding affinity for the 5-HT1A and 5-HT2A receptors, but is less potent in producing drug-appropriate responding, suggesting that the butyl compound has a higher efficacy at the receptor target. The drug discrimination assay for LSD in rats involves both 5-HT1A and 5-HT2A mediated components, and while lysergic acid 2-butyl amide is more potent than LSD as a 5-HT1A agonist, it is slightly less potent as a 5-HT2A agonist, and so would probably be slightly less potent than LSD as a hallucinogen in humans. The main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects, with the stereoselective activity of these unsymmetric monoalkyl lysergamides foreshadowing the subsequent development of compounds such as lysergic acid 2,4-dimethylazetidide (LSZ).
LSD art is any art or visual displays inspired by psychedelic experiences and hallucinations known to follow the ingestion of LSD. Artists and scientists have been interested in the effect of LSD on drawing and painting since it first became available for legal use and general consumption.

2-Bromo-LSD, also known as BOL-148, is a derivative of lysergic acid invented by Albert Hofmann, as part of the original research from which the closely related compound LSD was also derived. 2-Bromo-LSD was found to be inactive as a psychedelic and so was comparatively little researched for many years, although its similar behavior in the body made it useful for radiolabelling studies. It was found to bind to many of the same receptors as LSD, but acting as a neutral antagonist rather than an agonist. However its generally similar behavior to LSD in some respects has shown to be very useful in one specific area, the treatment of cluster headaches. These debilitating attacks have been known for some time to be amenable to treatment with certain hallucinogenic drugs such as LSD and psilocybin, but because of the illegal status of these drugs and the kind of mental changes they induce, research into their medical use has been slow and therapeutic application limited to very specific circumstances under strict supervision. It had been thought that this specific therapeutic action against cluster headaches was limited to hallucinogenic drugs of this type, and would always present a major barrier to their clinical use. However a serendipitous discovery found that 2-bromo-LSD is also able to produce this therapeutic effect, despite lacking the other effects of LSD. This has led to a resurgence of interest and research into 2-bromo-LSD and its possible medical uses. Some isolated incidents of hallucinogenic responses have been reported, but as with other non-hallucinogenic LSD analogues such as lisuride, this appears to be a rare side effect occurring only in individuals with an as yet unexplained susceptibility to this reaction. 2-Bromo-LSD reportedly attenuates the effects of LSD in humans.

Harris Isbell was an American pharmacologist and the director of research for the NIMH Addiction Research Center at the Public Health Service Hospital in Lexington, Kentucky from 1945 to 1963. He did extensive research on the physical and psychological effects of various drugs on humans. Early work investigated aspects of physical dependence with opiates and barbiturates, while later work investigated psychedelic drugs, including LSD. The research was extensively reported in academic journals such as the Journal of Pharmacology and Experimental Therapeutics, Psychopharmacologia, and the AMA Archives of Neurology and Psychiatry.
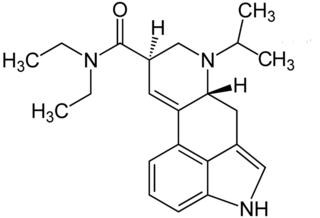
6-Isopropyl-6-nor-lysergic acid diethylamide (IP-LAD) is an analog of lysergic acid diethylamide (LSD) developed by the team of David E. Nichols. In studies on mice, it was found to be approximately 40% the potency of LSD, compared to the 60% increase in potency seen with ETH-LAD and roughly equivalent potency in AL-LAD and PRO-LAD.

1P-LSD or 1-propionyl-lysergic acid diethylamide is a psychedelic drug of the lysergamide class that is a derivative and functional analogue of LSD and a homologue of ALD-52. It has been sold online as a designer drug since 2015. It modifies the LSD molecule by adding a propionyl group to the nitrogen molecule of LSD's indole.
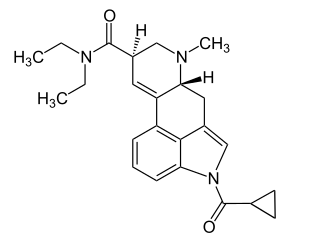
1cP-LSD is an acylated derivative of lysergic acid diethylamide (LSD), which has been sold as a designer drug. In tests on mice it was found to be an active psychedelic with similar potency to 1P-LSD.
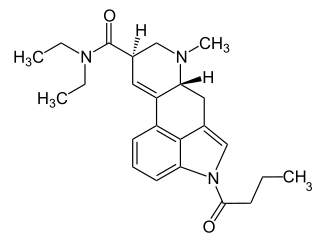
1B-LSD is an acylated derivative of lysergic acid diethylamide (LSD), which has been sold as a designer drug. In tests on mice it was found to be an active psychedelic, though with only around 1/7th the potency of LSD itself.



















