Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-borne diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".

Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampricide. The most common of these are herbicides, which account for approximately 50% of all pesticide use globally. Most pesticides are intended to serve as plant protection products, which in general, protect plants from weeds, fungi, or insects. As an example, the fungus Alternaria solani is used to combat the aquatic weed Salvinia.
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.

Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, ant chalk, and some products of Baygon in Southeast Asia.

Piperonyl butoxide (PBO) is a pale yellow to light brown liquid organic compound used as a synergist component of pesticide formulations. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. It is a semisynthetic derivative of safrole.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.
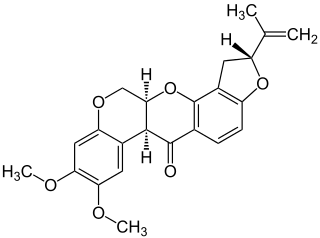
Rotenone is an odorless, colorless, crystalline isoflavone used as a broad-spectrum insecticide, piscicide, and pesticide. It occurs naturally in the seeds and stems of several plants, such as the jicama vine, and in the roots of several other members of the Fabaceae. It was the first-described member of the family of chemical compounds known as rotenoids.

Bifenthrin is a pyrethroid insecticide. It is widely used against ant infestations.
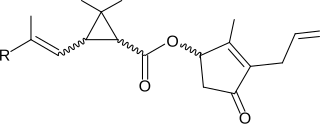
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums. Pyrethroids are used as commercial and household insecticides.

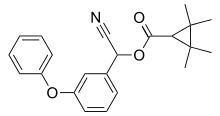
Permethrin is a medication and an insecticide. As a medication, it is used to treat scabies and lice. It is applied to the skin as a cream or lotion. As an insecticide, it can be sprayed onto outer clothing or mosquito nets to kill the insects that touch them.

Imidacloprid is a systemic insecticide belonging to a class of chemicals called the neonicotinoids which act on the central nervous system of insects. The chemical works by interfering with the transmission of stimuli in the insect nervous system. Specifically, it causes a blockage of the nicotinergic neuronal pathway. By blocking nicotinic acetylcholine receptors, imidacloprid prevents acetylcholine from transmitting impulses between nerves, resulting in the insect's paralysis and eventual death. It is effective on contact and via stomach action. Because imidacloprid binds much more strongly to insect neuron receptors than to mammal neuron receptors, this insecticide is more toxic to insects than to mammals.

The allethrins are a group of related synthetic compounds used in insecticides. They are classified as pyrethroids, i.e. synthetic versions of pyrethrin, a chemical with insecticidal properties found naturally in Chrysanthemum flowers. They were first synthesized in the United States by Milton S. Schechter in 1949. Allethrin was the first pyrethroid.

Deltamethrin is a pyrethroid ester insecticide. Deltamethrin plays a key role in controlling malaria vectors, and is used in the manufacture of long-lasting insecticidal mosquito nets; however, resistance of mosquitos and bed bugs to deltamethrin has seen a widespread increase.
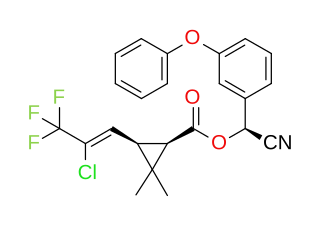
Cyhalothrin is the ISO common name for an organic compound that, in specific isomeric forms, is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as cyhalothrin are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.

The environmental effects of pesticides describe the broad series of consequences of using pesticides. The unintended consequences of pesticides is one of the main drivers of the negative impact of modern industrial agriculture on the environment. Pesticides, because they are toxic chemicals meant to kill pest species, can affect non-target species, such as plants, animals and humans. Over 98% of sprayed insecticides and 95% of herbicides reach a destination other than their target species, because they are sprayed or spread across entire agricultural fields. Other agrochemicals, such as fertilizers, can also have negative effects on the environment.

Clothianidin is an insecticide developed by Takeda Chemical Industries and Bayer AG. Similar to thiamethoxam and imidacloprid, it is a neonicotinoid. Neonicotinoids are a class of insecticides that are chemically similar to nicotine, which has been used as a pesticide since the late 1700s. Clothianidin and other neonicotinoids act on the central nervous system of insects as an agonist of nAChR, the same receptor as acetylcholine, the neurotransmitter that stimulates and activating post-synaptic acetylcholine receptors but not inhibiting AChE. Clothianidin and other neonicotinoids were developed to last longer than nicotine, which is more toxic and which breaks down too quickly in the environment.

Etofenprox is a pyrethroid derivative which is used as an insecticide. Mitsui Chemicals Agro Inc. is the main manufacturer of the chemical. It is also used as an ingredient in flea medication for cats and dogs.

Naled (Dibrom) is an organophosphate insecticide. Its chemical name is dimethyl 1,2-dibromo-2,2-dichloroethylphosphate.

Health effects of pesticides may be acute or delayed in those who are exposed. Acute effects can include pesticide poisoning, which may be a medical emergency. Strong evidence exists for other, long-term negative health outcomes from pesticide exposure including birth defects, fetal death, neurodevelopmental disorder, cancer, and neurologic illness including Parkinson's disease. Toxicity of pesticides depend on the type of chemical, route of exposure, dosage, and timing of exposure.

Tefluthrin is the ISO common name for an organic compound that is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as tefluthrin are often preferred as active ingredients in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. It is effective against soil pests because it can move as a vapour without irreversibly binding to soil particles: in this respect it differs from most other pyrethroids.