 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.440.630 |
| Chemical and physical data | |
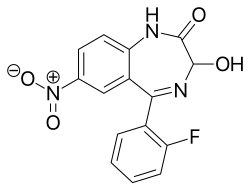
| Formula | C15H10FN3O4 |
| Molar mass | 315.260 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nifoxipam (3-hydroxydesmethylflunitrazepam, DP 370) is a benzodiazepine that is a minor metabolite of flunitrazepam and has been sold online as a designer drug. [1] [2] [3] [4] [5] [6] [7] [8] [9]
Nifoxipam produces strong tranquillising and sleep-prolonging effects and has much lower toxicity compared to lormetazepam and flunitrazepam in mice. [1]