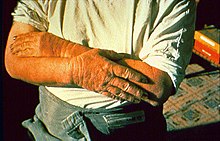
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can vary from small to covering the entire body. Dermatitis is often called eczema, and the difference between those terms is not standardized.

Irritation, in biology and physiology, is a state of inflammation or painful reaction to allergy or cell-lining damage. A stimulus or agent which induces the state of irritation is an irritant. Irritants are typically thought of as chemical agents but mechanical, thermal (heat), and radiative stimuli can also be irritants. Irritation also has non-clinical usages referring to bothersome physical or psychological pain or discomfort.

p-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna.

Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes are not contagious or life-threatening, but can be very uncomfortable.

White spirit (AU, UK and Ireland) or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ), turpentine substitute, and petroleum spirits, is a petroleum-derived clear liquid used as a common organic solvent in painting. There are also terms for specific kinds of white spirit, including Stoddard solvent and solvent naphtha (petroleum). White spirit is often used as a paint thinner, or as a component thereof, though paint thinner is a broader category of solvent. Odorless mineral spirits (OMS) have been refined to remove the more toxic aromatic compounds, and are recommended for applications such as oil painting.

Chemical hazards are typical of hazardous chemicals and hazardous materials in general. Exposure to certain chemicals can cause acute or long-term adverse health effects. Chemical hazards are usually classified separately from biological hazards (biohazards). Main classifications of chemical hazards include asphyxiants, corrosives, irritants, sensitizers, carcinogens, mutagens, teratogens, reactants, and flammables. In the workplace, exposure to chemical hazards is a type of occupational hazard. The use of protective personal equipment (PPE) may substantially reduce the risk of damage from contact with hazardous materials.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
An occupational disease or industrial disease is any chronic ailment that occurs as a result of work or occupational activity. It is an aspect of occupational safety and health. An occupational disease is typically identified when it is shown that it is more prevalent in a given body of workers than in the general population, or in other worker populations. The first such disease to be recognised, squamous-cell carcinoma of the scrotum, was identified in chimney sweep boys by Sir Percival Pott in 1775. Occupational hazards that are of a traumatic nature are not considered to be occupational diseases.

A patch test is a diagnostic method used to determine which specific substances cause allergic inflammation of a patient's skin.
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
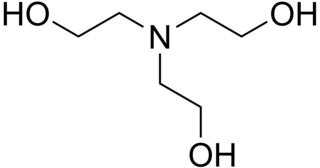
Triethanolamine, or TEOA, is an organic compound with the chemical formula N(CH2CH2OH)3. It is a colourless, viscous liquid. It is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although samples may appear yellow because of impurities.

Perioral dermatitis, also known as periorificial dermatitis, is a common type of skin rash. Symptoms include multiple small (1–2 mm) bumps and blisters sometimes with background redness and scale, localized to the skin around the mouth and nostrils. Less commonly the eyes and genitalia may be involved. It can be persistent or recurring and resembles particularly rosacea and to some extent acne and allergic dermatitis. The term "dermatitis" is a misnomer because this is not an eczematous process.
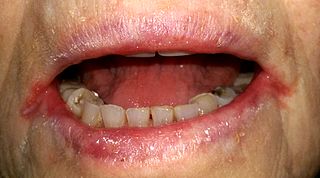
Angular cheilitis (AC) is inflammation of one or both corners of the mouth. Often the corners are red with skin breakdown and crusting. It can also be itchy or painful. The condition can last for days to years. Angular cheilitis is a type of cheilitis.

Allergic contact dermatitis (ACD) is a form of contact dermatitis that is the manifestation of an allergic response caused by contact with a substance; the other type being irritant contact dermatitis (ICD).
Irritant contact dermatitis is a form of contact dermatitis that can be divided into forms caused by chemical irritants and those caused by physical irritants.
A barrier cream is a topical formulation used in industrial applications and as a cosmetic to place a physical barrier between the skin and contaminants that may irritate the skin. There are many other terms for creams designed to protect skin from harmful substances, including skin protective creams, pre-work creams, antisolvent gels, protective ointments, and shielding lotions. Three classes of barrier creams are used: water repellent creams, water-soluble creams, and creams designed for special applications. Barrier creams may contain substances such as zinc oxide, talc or kaolin to layer over the skin. For hand care they are designed to protect against the harm from detergents and other irritants.
Occupational skin diseases are ranked among the top five occupational diseases in many countries.
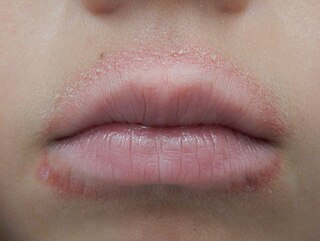
Lip licker's dermatitis is a type of skin inflammation around the lips due to damage by saliva from repetitive lip licking and is classified as a subtype of irritant contact cheilitis. The resulting scaling, redness, chapping, and crusting makes a well-defined ring around the lips. The rash may extend as far as the tongue can reach and usually does not occur at the corners of the mouth. It commonly occurs during winter months but some people can have it year-round if lip licking is a chronic habit.
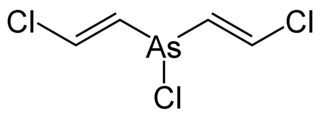
Lewisite 2(L-2) is an organoarsenic chemical weapon with the formula AsCl(CH=CHCl)2. It is similar to lewisite 1 and lewisite 3 and was first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride (lewisite 1) as well as bis(2-chloroethenyl) arsinous chloride (lewisite 2) and tris(2-chlorovinyl)arsine (lewisite 3). Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.

Lewisite 3(L-3) is an organoarsenic chemical weapon like lewisite 1 and lewisite 2 first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride as well as bis(2-chloroethenyl) arsinous chloride and tris(2-chlorovinyl)arsine. Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.