 | |
| Names | |
|---|---|
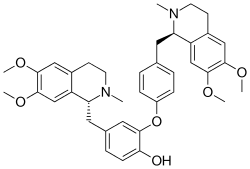
| Preferred IUPAC name (11R,71R)-16,17,76,77-Tetramethoxy-12,72-dimethyl-11,12,13,14,71,72,73,74-octahydro-4-oxa-1,7(1)-diisoquinolina-3(1,3),5(1,4)-dibenzenaheptaphan-34-ol | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.622 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C38H44N2O6 | |
| Molar mass | 624.778 g·mol−1 |
| Density | 1.186 g/mL |
| Melting point | 115 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dauricine is a plant metabolite, chemically classified as a phenol, an aromatic ether, and an isoquinoline alkaloid. [1] It has been isolated from the Asian vine Menispermum dauricum , Asian moonseed, and the North American vine Menispermum canadense , Canadian moonseed. [2] Scientists Tetsuji Kametani and Keiichiro Fukumoto of Japan are credited with being the first to synthesize dauricine in 1964, using both the Arndt-Eistert reaction and Bischler-Napieralski reaction to do so. [3] Dauricine has been studied in vitro for its potential to inhibit cancer cell growth [4] [5] [6] [7] and to block cardiac transmembrane Na+, K+, and Ca2+ ion currents. [8]