This article needs additional citations for verification .(July 2019) |
A potassium channel opener is a type of drug which facilitates ion transmission through potassium channels.
This article needs additional citations for verification .(July 2019) |
A potassium channel opener is a type of drug which facilitates ion transmission through potassium channels.
Some examples include:
| Class | Subclasses | Activators |
|---|---|---|
| Calcium-activated | [ citation needed ]
| |
| Inwardly rectifying |
| [ citation needed ]
|
| [ citation needed ]
| |
| [ citation needed ] | |
| Tandem pore domain | [ citation needed ] | |
| Voltage-gated |
|
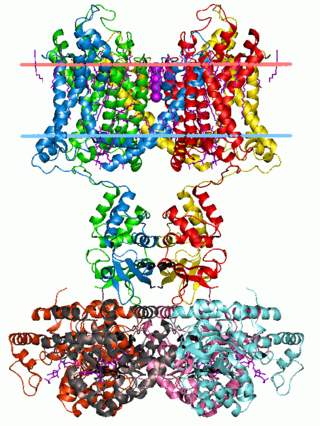
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.

Nicorandil is a vasodilator drug used to treat angina.

The two-pore-domain or tandem pore domain potassium channels are a family of 15 members that form what is known as leak channels which possess Goldman-Hodgkin-Katz (open) rectification. These channels are regulated by several mechanisms including signaling lipids, oxygen tension, pH, mechanical stretch, and G-proteins. Two-pore-domain potassium channels correspond structurally to a inward-rectifier potassium channel α-subunits. Each inward-rectifier potassium channel α-subunit is composed of two transmembrane α-helices, a pore helix and a potassium ion selectivity filter sequence and assembles into a tetramer forming the complete channel. The two-pore domain potassium channels instead are dimers where each subunit is essentially two α-subunits joined together.

Kv7.2 (KvLQT2) is a voltage- and lipid-gated potassium channel protein coded for by the gene KCNQ2.

Kv7.3 (KvLQT3) is a potassium channel protein coded for by the gene KCNQ3.
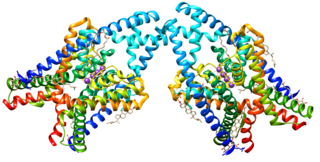
Potassium channel subfamily K member 3 is a protein that in humans is encoded by the KCNK3 gene.

Potassium voltage-gated channel subfamily KQT member 4, also known as voltage-gated potassium channel subunit Kv7.4, is a protein that in humans is encoded by the KCNQ4 gene.

Potassium channel subfamily K member 1 is a protein that in humans is encoded by the KCNK1 gene.

Potassium channel subfamily K member 4 is a protein that in humans is encoded by the KCNK4 gene. KCNK4 protein channels are also called TRAAK channels.

Potassium channel subfamily K member 5 is a protein that in humans is encoded by the KCNK5 gene.

G protein-activated inward rectifier potassium channel 3 is a protein that in humans is encoded by the KCNJ9 gene.

Potassium channel subfamily K member 17 is a protein that in humans is encoded by the KCNK17 gene.

Potassium channel, subfamily K, member 7, also known as KCNK7 or K2P7.1 is a protein which is encoded in humans by the KCNK7 gene. K2P7.1 is a potassium channel containing two pore-forming P domains. Multiple transcript variants encoding different isoforms have been found for this gene.

Potassium channel, subfamily K, member 10, also known as KCNK10 is a human gene. The protein encoded by this gene, K2P10.1, is a potassium channel containing two pore-forming P domains.

Potassium channel subfamily K member 16 is a protein that in humans is encoded by the KCNK16 gene. The protein encoded by this gene, K2P16.1, is a potassium channel containing two pore-forming P domains.

Potassium channel subfamily K member 18 (KCNK18), also known as TWIK-related spinal cord potassium channel (TRESK) or K2P18.1 is a protein that in humans is encoded by the KCNK18 gene. K2P18.1 is a potassium channel containing two pore-forming P domains.
Potassium channel blockers are agents which interfere with conduction through potassium channels.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.
Michel Lazdunski is a French biologist specializing in biochemistry, physiology, pathophysiology, molecular pharmacology and neuroscience.
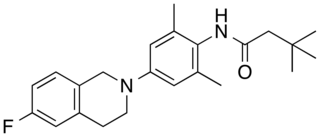
XEN1101 (encukalner) is an experimental small molecule anticonvulsant and selective Kv7.2/Kv7.3 potassium channel opener being investigated as a treatment for refractory focal onset seizures and major depressive disorder.
XEN1101 is a novel positive allosteric modulator ("opener") of the potassium channel KCNQ2/3 (Kv7.2/7.3) currently being developed by Xenon Pharmaceuticals Inc. for the treatment of focal epilepsy.