
Rhubarb is the fleshy, edible stalks (petioles) of species and hybrids of Rheum in the family Polygonaceae, which are cooked and used for food. The plant is a herbaceous perennial that grows from short, thick rhizomes. Historically, different plants have been called "rhubarb" in English. The large, triangular leaves contain high levels of oxalic acid and anthrone glycosides, making them inedible. The small flowers are grouped in large compound leafy greenish-white to rose-red inflorescences.

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Polyphenols are a large family of naturally occurring phenols. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

Vigna umbellata, previously Phaseolus calcaratus, is a warm-season annual vine legume with yellow flowers and small edible beans. It is commonly called ricebean or rice bean. To date, it is little known, little researched and little exploited. It is regarded as a minor food and fodder crop and is often grown as intercrop or mixed crop with maize, sorghum or cowpea, as well as a sole crop in the uplands, on a very limited area. Like the other Asiatic Vigna species, ricebean is a fairly short-lived warm-season annual. Grown mainly as a dried pulse, it is also important as a fodder, a green manure and a vegetable. Ricebean is most widely grown as an intercrop, particularly of maize, throughout Indo-China and extending into southern China, India, Nepal and Bangladesh. In the past it was widely grown as lowland crop on residual soil water after the harvest of long-season rice, but it has been displaced to a great extent where shorter duration rice varieties are grown. Ricebean grows well on a range of soils. It establishes rapidly and has the potential to produce large amounts of nutritious animal fodder and high quality grain.

Rhaphiolepis is a genus of about fifteen species of evergreen shrubs and small trees in the family Rosaceae, native to warm temperate and subtropical East Asia and Southeast Asia, from southern Japan, southern Korea and southern China, south to Thailand and Vietnam. In searching literature it is well to remember that the name commonly is misspelt "Raphiolepsis". The genus is closely related to Eriobotrya (loquats), so closely in fact, that members of the two genera have hybridised with each other; for example the "Coppertone loquat" is a hybrid of Eriobotrya deflexa X Rhaphiolepis indica. The common name hawthorn, originally specifically applied to the related genus Crataegus, now also appears in the common names for some Rhaphiolepis species. For example, Rhaphiolepis indica often is called "Indian hawthorn", and Rhaphiolepis umbellata, "Yeddo hawthorn".

Rhaphiolepis umbellata is a species of flowering plant in the family Rosaceae, native to Korea, Japan and Taiwan. Growing to 1.5 m (5 ft) tall and wide, it is an evergreen shrub with glossy oval leaves, and scented white flowers, sometimes tinged with pink, in early summer.

Eucalyptus perriniana, commonly known as spinning gum, is a tree or mallee which is native to New South Wales, the Australian Capital Territory, Victoria and Tasmania. Spinning gum is a sub-alpine species and grows in areas which are normally snow covered for several months in winter. However domestic cultivars can grow in almost any temperate climate.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

The color of wine is one of the most easily recognizable characteristics of wines. Color is also an element in wine tasting since heavy wines generally have a deeper color. The accessory traditionally used to judge the wine color was the tastevin, a shallow cup allowing one to see the color of the liquid in the dim light of a cellar. The color is an element in the classification of wines.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.
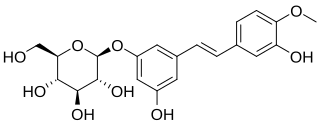
Rhaponticin is a stilbenoid glucoside compound. Its aglycone is called rhapontigenin. It can be found in rhubarb rhizomes.

The phenolic content in tea refers to the phenols and polyphenols, natural plant compounds which are found in tea. These chemical compounds affect the flavor and mouthfeel of tea. Polyphenols in tea include catechins, theaflavins, tannins, and flavonoids.

Bergenia ciliata is a plant species in the genus Bergenia, deciduous in USDA Zones 5 to 7, but usually remain semi-evergreen south of Zone 7. It is found in Northern India in Uttarakhand and Himachal Pradesh. This flower is related to the famous Phool Dei Festival celebrated in Uttarakhand. It is commonly known in India as Pathar phor buti. Also found in mountain areas of West Bengal, like Kalimpong, and Darjeeling. Afghanistan, south Tibet, Northern Nepal, Bhutan.

Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct. Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.
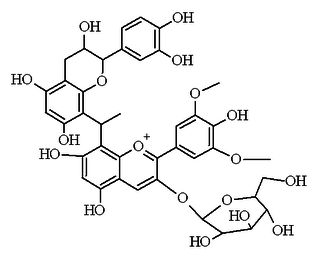
Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.

Catechin-7-O-glucoside is a flavan-3-ol glycoside formed from catechin.

Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone. It is a C-3 methyl substituted chrysazin of the trihydroxyanthraquinone family.


















