
Selenium is a chemical element with the symbol Se and atomic number 34. It is a metalloid with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Selenocysteine is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur.

Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, only L-cysteine is found in nature.

Glutathione peroxidase (GPx) is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydroperoxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
In molecular biology a selenoprotein is any protein that includes a selenocysteine amino acid residue. Among functionally characterized selenoproteins are five glutathione peroxidases (GPX) and three thioredoxin reductases, (TrxR/TXNRD) which both contain only one Sec. Selenoprotein P is the most common selenoprotein found in the plasma. It is unusual because in humans it contains 10 Sec residues, which are split into two domains, a longer N-terminal domain that contains 1 Sec, and a shorter C-terminal domain that contains 9 Sec. The longer N-terminal domain is likely an enzymatic domain, and the shorter C-terminal domain is likely a means of safely transporting the very reactive selenium atom throughout the body.

Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase, is a pyridoxal phosphate (PLP)-dependent transaminase enzyme that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT level, and their ratio are commonly measured clinically as biomarkers for liver health. The tests are part of blood panels.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.
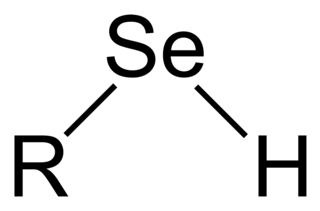
Selenols are organic compounds that contain the functional group with the connectivity C–Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known selenol is the amino acid selenocysteine.
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:

Glutathione peroxidase 1, also known as GPx1, is an enzyme that in humans is encoded by the GPX1 gene on chromosome 3. This gene encodes a member of the glutathione peroxidase family. Glutathione peroxidase functions in the detoxification of hydrogen peroxide, and is one of the most important antioxidant enzymes in humans.

Glutathione peroxidase 4, also known as GPX4, is an enzyme that in humans is encoded by the GPX4 gene. GPX4 is a phospholipid hydroperoxidase that protects cells against membrane lipid peroxidation.

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction

The enzyme methionine γ-lyase (EC 4.4.1.11, MGL) is in the γ-family of PLP-dependent enzymes. It degrades sulfur-containing amino acids to α-keto acids, ammonia, and thiols:

In enzymology, a 3-mercaptopyruvate sulfurtransferase is an enzyme that catalyzes the chemical reactions of 3-mercaptopyruvate. This enzyme belongs to the family of transferases, specifically the sulfurtransferases. This enzyme participates in cysteine metabolism. It is encoded by the MPST gene.
The enzyme sulfinoalanine decarboxylase (EC 4.1.1.29) catalyzes the chemical reaction

Selenide, water dikinase 1 is an enzyme that in humans is encoded by the SEPHS1 gene.

15 kDa selenoprotein is a protein that in humans is encoded by the SEP15 gene. Two alternatively spliced transcript variants encoding distinct isoforms have been found for this gene.

Although it is toxic in large doses, selenium is an essential micronutrient for animals. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).

In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.


















